Abstract
Background
IgA and its secretory form sIgA impact protection from infection and necrotising enterocolitis but little is known about quantities in preterm mums own milk (MOM) or infant stool, onset of endogenous production in the preterm gut, and what affects these.
Methods
We measured by ELISA in MOM and stool from healthy preterm infants total IgA and sIgA longitudinally and additionally in MOM fresh, refrigerated, frozen, and after traversing feeding systems.
Results
In 42 MOM (median gestation 26 weeks), we showed total IgA levels and sIgA were highest in colostrum, fell over 3 weeks, and were not impacted by gestation. Median IgA values matched previous term studies (700 mcg/ml). In MOM recipients stool IgA was detected in the first week, at around 30% of MOM quantities. Formula fed infants did not have detectable stool IgA until the third week. Levels of IgA and sIgA were approximately halved by handling processes.
Conclusions
MOM in the 3 weeks after preterm delivery contains the highest concentrations of IgA and sIgA. Endogenous production after preterm birth occurs from the 3 week meaning preterm infants are dependent on MOM for IgA which should be optimised. Routine NICU practices halve the amount available to the infant.
Impact
-
(Secretory) Immunoglobulin A (IgA) is present in colostrum of maternal milk from infants as preterm as 23–24 weeks gestational age, falling over the first 3 weeks to steady levels similar to term.
-
Gestation at birth does not impact (secretory) IgA levels in breast milk.
-
IgA is present in very preterm infant stools from maternal milk fed infants from the first week of life, but not in formula milk fed preterm infants until week three, suggesting endogenous production from this point.
-
Refrigeration, freezing, and feeding via plastic tubing approximately halved the amount of IgA available.
Similar content being viewed by others
Introduction
Mother’s own milk (MOM) is optimal nutrition for all newborn infants particularly preterm infants at risk of necrotising enterocolitis (NEC) and sepsis.1 In addition to nutrients for growth and development, MOM contains bioactive components that impact short and long-term health.2 Interactions between these components modulate microbial activity and educate the naïve immune system.3 MOM contains high concentrations of immunomodulatory factors, including immunoglobulin, especially immunoglobulin A (IgA), cytokines, growth factors, human milk oligosaccharides, lactoferrin, and enzymes such as lysozyme.4 These foster initial development of the infant’s immune system, promoting balanced pro- and anti-inflammatory responses, immunity and tolerance.5
Secretory IgA (sIgA) is in both breast milk and mucosal secretions and is critical in exclusion of pathogens and education of the immune system.6 sIgA constitutes dimeric or polymeric IgA bound by a “J” chain, complexed to secretory component (SC).7 The SC binds to dimeric IgA within the intestinal epithelium during membrane transcytosis utilising a glycoprotein, polymeric Ig receptor.2 It is hypothesised that this SC delays proteolysis and digestion in the gut lumen.7 Demers-Mathieu et al. (2019) demonstrated IgA found in 20 preterm infants (mean gestational age (GA)30 weeks) stool is only 1% sIgA.8
Endogenous production of secretory immunoglobulin does not occur immediately after birth and infants are reliant on maternal transfer.5 Term infants rely on placental transfer of immunoglobulin (as IgG) primarily in the third trimester meaning significantly preterm infants miss out on this passive transfer of other forms of immunoglobulin. Breast fed infants acquire their sIgA via MOM until endogenous production starts. Extremely preterm infants are unable to directly breast feed and reliant on MOM being expressed, and often stored refrigerated or frozen until tolerated. Some infants receive donor breast milk (DBM) which in addition to being expressed and stored like MOM is also pasteurised. sIgA in DBM is reduced by pasteurisation9 but little data exist on the impact of storage and handling procedures on (s)IgA in MOM.
Endogenous IgA production may commence at set postnatal age, GA, or a combination. Studies to date mainly focus on term infants,10 with little known about endogenous sIgA production in the significantly preterm infant. However, this is of key importance to better understand and manage their susceptibility to infection and mucosal inflammation, as NEC and late onset sepsis (LOS) remain important causes of death and serious morbidity after preterm birth. Although the pathogenesis remains incompletely understood NEC is strongly associated with inflammation, mucosal damage and LOS with translocation of gut organisms into systemic circulation. A lack of endogenous mucosal sIgA may contribute to an impaired ability to bind and excrete pathogens, leaving preterm infants susceptible.11,12
Little is also understood about the impact of current storage practices on concentrations of (s)IgA in MOM. In a cohort of significantly preterm infants and their mothers this study aimed to (1) describe the impact on IgA levels in expressed MOM of refrigerating, freezing and feeding via a nasogastric tube (NG) tube, (2) longitudinally quantify total IgA and sIgA in maternal milk and infant stool, and (3) determine differences between exclusively MOM or formula fed infants, in a cohort of exclusively healthy preterm infants (<32 weeks GA).
Methods
Participants and sample collection
Participants were recruited to an ongoing REC approved sample salvage study (SERVIS Supporting Research in Vulnerable Infants REC 10/H0908/39) of >900 babies <32 weeks GA. Eligible infants were cared for in the Neonatal Intensive Care Unit (NICU) at the Royal Victoria Infirmary, Newcastle. We developed Standard Operating Procedures (SOPs) for collection of infant stool and MOM. In brief, the bedside nurse collected stool samples daily from the nappy into a sterile container at −20 °C. Samples were anonymised before being transported weekly to a −80 °C freezer. MOM was sampled from the residual milk in systems used to feed babies to prevent loss of milk volume to the baby. MOM sample day thus identifies day of life milk received by infant, and is not necessarily the day it was expressed.
In addition, MOM was obtained fresh from ten mothers of different preterm infants and for each sample analysis was performed on fresh, refrigerated and frozen milk, and after fresh milk was administered via continuous pump and syringe (n = 40 total analyses).
Longitudinal samples were selected from 42 best-matched healthy preterm infants defined as those who did not develop NEC, LOS or focal perforation, and survived to discharge (Fig. 1). Demographics and sampling data are given in Table 1. For multiple births only one infant was studied. Milk samples were analysed across six time points in the first 120 days of life chosen to reflect colostrum, early milk, transitional milk, and mature breastmilk expression. A single sample per subject was used in each time point to avoid problems with repeated measures. Six additional healthy preterm infants who only received formula milk throughout their stay, in whom we had longitudinal stool sampling, were also selected.
Sample preparation and ELISA
Samples were thawed on ice and the total IgG, IgM, IgA, and sIgA components of breast milk (and total IgA and sIgA from infant stool) were quantified using in-house sandwich enzyme-linked immunosorbent assays (ELISA) using standardised protocols.
Wells of 96 well microtitre plates (Immulon 4HBX, Fisher Scientific UK Ltd) were coated with 100 µl of capture antibodies at 1 µg/ml in Coating Buffer (35 mM NaHCO3, 15 mM Na2CO3 pH 9.6) and incubated overnight at 4 °C. Capture antibodies were anti-human IgG (γ-chain); anti-human IgM (μ-chain) or anti-human IgA (α-chain) (all Sigma).
Plates were then washed with PBS containing 0.05% (v/v) Tween 20 (PBSTw) on a plate washer and non-specific sites were blocked by incubation with 150 µl/well PBS containing 5% (w/v) (PBSA) for 45 min at room temperature.
After washing with PBSTw, 100 µl/well of sample or antibody standard (diluted in PBSTw containing 0.5% (w/v) (PBSATw) was added and incubated at room temperature for 2 h.
Standards used were IgG from human serum (Sigma I2511); IgM from human serum (Sigma I8260), or IgA from human colostrum (Sigma I2636). A broad standard curve was prepared ranging from 5000 to 0.5 ng/ml.
Following a further two washes with PBSTw, bound IgG, IgM or IgA were detected in a 45 min incubation with 100 µl of secondary goat anti-human IgG, IgM, or IgA peroxidase-conjugated antibodies (IgA) (all Sigma) respectively, added at a 1:5000 dilution.
To specifically determine the sIgA component of IgA, an additional step followed the standard/sample incubation. Mouse anti-human IgA secretory chain antibody (Sigma I6635) was added (100 µl/well at 1:10000) for 1 h and after washing, mouse anti-human IgG peroxidase-conjugated antibody (Sigma) was added 100 µl/well at 1:1000.
Following two further wash cycles, bound peroxidase activity was determined using 100 µl/well Developer (o-phenylenediamine (OPD) 4 mM in 0.1 M Citrate Buffer pH 5.0 containing 0.012% (v/v) H2O2. The colour was allowed to develop for 5 min prior to stopping the reaction with 100 µl/well Stop Buffer (2 M H2SO4).
Absorbance at 492 nm was measured on a BioTek MRXII plate reader (Agilent Technologies, Harwell UK). GraphPad Prism™ software was used to create a standard curve with a logistic curve fit and immunoglobulin concentrations were measured using interpolated data.
Statistical analysis
Mann–Whitney tests for unpaired sample comparisons (across maternal milk fed infants and formula milk fed infants) were applied using GraphPad Prism™ and R studio. Differences in longitudinal IgA and sIgA concentrations were analysed using Kruskal–Wallis tests for pairwise comparisons. Linear regression models were applied using R statistical software (version 1.4.1717), using the emmeans package for post hoc linear regression analysis between groups, and Dharma for testing residuals, to determine if changes in concentrations of IgA and sIgA over time of expression remained significant when adjusted for potentially confounding variables.
Results
IgA and sIgA are reduced with standard milk storage and feeding practices on neonatal intensive care
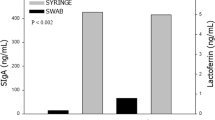
In the ten fresh samples we demonstrated IgA and sIgA levels were reduced to below half the baseline level of the original sample by refrigerating, freezing, or feeding via continuous syringe feed (fresh median IgA 1859–562 µg/ml (combined refrigerated, frozen and syringe fed), fresh median sIgA 1569–740 µg/ml (combined), p < 0.05) (Fig. 2a, b). IgG and IgM levels were not impacted by the different milk storage and feeding practices (all p > 0.05; Fig. 2c, d).
Longitudinal total IgA and sIgA in maternal breast milk and impact of gestation
In MOM of 42 healthy preterm infants, total IgA and sIgA concentrations were highest in milk received in the first week of life, ranging from 1047 to 1866 µg/ml (median 1488 µg/ml) then gradually reduced to a stable concentration in milk received in and beyond the 3 week of life of around 650 µg/ml (Fig. 3a, b). Apart from day of life, no infant or maternal factors that we had access to influenced IgA or sIgA concentration including sex, delivery mode, antenatal steroid receipt, multiple pregnancy or chorioamnionitis. (Supplementary Table 1). Using data from all infants longitudinally, plotted against corrected gestational age (CGA), as CGA increased, the total IgA and sIgA in MOM reduced (p < 0.0001) (Fig. 3c, d).
Concentration of (a) total Immunoglobulin A and (b) secretory immunoglobulin A as day of life increased. n = 42. Concentration of (c) total and (d) secretory immunoglobulin A in cohorts of two-weekly gestational age, n = number of samples in each time cohort. Data depicted as Box and Whisker plots showing median and range. Single sample per subject included within each time point (n = 42). Statistically significant variables shown using Mann–Whitney test.
Because of the overall impact of day of life on MOM IgA levels we explored the impact of GA on samples fed on days 0–7 separately from those fed later (Fig. 4) using ANOVA. This demonstrated that birth gestation did not have a statistically significant influence on total IgA and sIgA. IgA was detectable in colostrum in even the most immature infants born as early as 23- and 24-weeks gestation, with levels in these day 0–7 samples higher than the median for all samples (1401 µg/ml (IQR 1261–1532 µg/ml) versus 703 µg/ml (IQR 523–930 µg/ml)) (Fig. 4a, b). Similarly, after the first week of life gestation at birth did not impact IgA or sIgA (Fig. 4c, d).
First week of life (a) and (b) and beyond first week of life (c) and (d). Concentration of total Immunoglobulin A (a) and (c) and Secretory Immunoglobulin A (b) and (d) in maternal milk by gestational age at birth. Single sample per subject included within each time point. Data depicted as Box and Whisker plots showing median and range n = 42. No statistical differences in any analysis.
Impact of maternal milk on IgA and sIgA in infant stool
Longitudinal analysis of stool showed IgA and sIgA were detectable in the stool of breast-milk-fed preterm infants from the first week of life (IgA median concentration 403 µg/ml, IQR 162–625 µg/ml, sIgA 91 µg/ml, IQR 7–138 µg/ml) increasing with increasing postnatal age (IgA median concentration after 1 month of life ~1000 µg/ml) (Fig. 5a, b). sIgA concentration correlated to ~25–30% of IgA concentration. However, when this data is represented by CGA no significant difference is seen in stool IgA with time (p 0.13) (Fig. 5c, d). Comparing MOM with corresponding day of life stool over the first 21 days, correlation was demonstrated (Pearson correlation r = 0.45 (95% confidence interval −0.06–0.77 p = 0.082)), but no correlation was seen after this point (Pearson r = 0.006 (95% confidence interval –0.3–0.3 p = 0.97) (Supplementary Fig. 2).
a Total Immunoglobulin A concentration and (b) Secretory immunoglobulin A concentration by DoL of sample. c Total Immunoglobulin A concentration and (d) Secretory immunoglobulin A concentration expressed by corrected gestational age. Single sample per subject included within each time point. Data depicted as Box and Whisker plots with median and range n = 36. Statistically significant differences tested with Mann–Whitney.
Total IgA and sIgA in formula fed infant stool
In the six infants exclusively fed formula milk, IgA and sIgA were not detectable in infant stool until 17 days of age at the earliest (range 17–47 days). This corresponded to an earliest CGA of 31 weeks. Median detection of IgA occurred at 37 (IQR 31–45) days of life and median CGA 34 (IQR 31–35) weeks (Fig. 6a, b). When infant stool was compared between MOM fed infants and formula milk fed infants, there was a significant difference (p < 0.05) between total IgA levels over the first month of life (Fig. 6c). In a similar comparison using CGA, the concentration of total IgA in stool from MOM fed infants remained static but in stool from formula fed infants IgA increased significantly in the small number of infants sampled beyond 34 weeks CGA (p < 0.005) (Fig. 6d). Comparison of stool IgA in MOM fed infants with formula fed infants suggests endogenous production from DOL 30 onwards is likely to be contributing to levels in MOM fed infants from this point onwards (Fig. 6c, d).
a Total immunoglobulin A concentration in infant stools from preterm formula milk fed infants expressed by day of life. b Total immunoglobulin A concentration in infant stools from formula milk fed infants expressed by corrected gestational age. Data depicted as mean. n = 6. c and d Concentration of total immunoglobulin A in infant stool in maternal milk fed infants (n = 42) compared with formula milk fed infants (n = 6) expressed by day of life (c) and corrected gestational age (d). Data depicted as Box and Whisker plots with median and range.
Discussion
We provide data on total IgA and sIgA concentration in MOM and infant stool, in the largest, most immature cohort of healthy preterm infants published to date. We have shown that IgA and sIgA are highest in colostrum even at gestations as low as 23 weeks and gradually reduce over the first three weeks. In infant stool IgA was detectable during the first week of life in infants receiving MOM with levels correlating to those in MOM in the first three weeks of life but not beyond this. We demonstrated absence of stool IgA in formula fed infants until at least day 17, and an increase in IgA/sIgA in infant stool beyond this, giving insight into the postnatal age and gestation at which endogenous IgA production occurs in the significantly preterm infant. We also demonstrated significant and potentially clinically important loss of total and sIgA in MOM samples that were either refrigerated, frozen or salvaged from feeding lines.
Milk: In term MOM the concentration of IgA in human breastmilk varies from <1 g/l to 12 g/l in colostrum, reducing to a more consistent 0.5 g/l in mature milk (over a month of life).13,14,15,16,17,18,19,20 Our overall median of 700 µg/ml is in keeping with this. Previous studies have limited breastmilk samples from mothers delivering very immature infants (median GA of all previous preterm studies 28–31 weeks in comparison to ours of 26 weeks),13,14,15,16,17,18 have limited access to the earliest milk samples,21,22 and have fewer preterm infants (15–30).
Older studies (1980’s) using immunoelectrophoresis to quantify total IgA in breastmilk identified higher concentrations of IgA in milk from preterm (median GA 31 and 30 weeks respectively) compared with term mothers,14,18 not affected by expression. Use of immunoblotting to determine sIgA levels showed no significant difference in sIgA concentration in preterm compared with term MOM.16 Hsu et al. (2014) used ELISA in MOM from 17 preterm infants (median GA 30 weeks) identifying no difference in actual values or trends over time compared to term MOM,15 confirmed by Trend et al. (2016) analysing with ELISA MOM samples from 30 preterm mothers (median GA 28 weeks) over the first month of life.13
Our finding that IgA concentration decreases with increasing postnatal age is in keeping with previously published literature, with total IgA concentration decreasing from colostrum to mature milk in preterm-delivering maternal breast milk, and a similar concentration of total IgA in preterm and term delivering mothers at older postnatal ages.18
Stool: The median concentration of IgA in preterm infant stool was 370 (162–625) µg/mg in the first week of life increasing to 1000 µg/mg by a month of age. Very little data exist to compare these results to, with a review of the literature revealing only two studies quantifying sIgA in breast milk fed preterm infant stool.8,23 In term infants, literature has previously described sIgA measurable in MOM fed infants within the first week of life.10
Preterm infant work published by Demers-Mathieu et al. (2019) found that in a cohort of twenty preterm infants (30 ± 3 weeks), 85% of immunoglobulin in infant stool was total IgA of which only 1% was sIgA.21 They hypothesise that this is due to proteolytic cleavage of the SC during digestion in the infant gut. We found up to 35% as much sIgA as total IgA in infant stool, possibly due to improved sIgA detection techniques in this study, where we aimed to first isolate IgA then subsequently SC, and thus identifying specifically sIgA rather than a composite of sIgA, sIgM and SC as has been done in previous studies. Lower gastric acidity in preterm infants may mean digestion of sIgA may be reduced in preterm infants compared with term.8
The postnatal age at which preterm infants begin to produce endogenous sIgA is not well described in current literature. Intestinal IgA plasma cells are not readily detected in the lamina propria until after a month of life in term infants,24 and they do not reach adult levels until 2 years of life.25 In saliva sIgA is detected in term infants by 1 month of age.26 Mice begin to actively generate intestinal sIgA only after weaning but young mice lacking maternal sIgA from milk generate active intestinal sIgA from a younger age,27 suggesting that in mice at least endogenous production is influenced by diet and not fixed. These results are in keeping with the negligible IgA in formula fed infants stools until 17 days of life at the earliest and similar findings have been documented before. Eibl et al. (1988) measured total faecal IgA in 13 preterm formula milk fed infants (gestation unknown, birth weight 1000–2000g) of whom half were treated with oral IgA. In control infants (not fed oral IgA) no faecal IgA was detected before 4 weeks of age but those fed exogenous IgA had between 1 and 10 mg/g of IgA measurable in stool.28 A recent study determined bacterial binding of IgA in preterm infants’ stool and found that in those who were formula fed in the first month of life, <1% of bacteria were bound by IgA.23
This study presents data from the largest most immature preterm cohort to date and includes 23 and 24 week MOM and stool. All infants were known to be healthy as defined by rigorous criteria, and drawn from a much bigger dataset from which infants not fulfilling these criteria were excluded. Our cohort benefits from analysis of MOM and infant stool samples both early in life (28 samples in first week) and longitudinally (average sampling up to 8 weeks). In addition, standardised sampling, storage and analysis SOP’s were applied allowing for robust analysis. Owing to the large overall cohort, there was availability of well-matched stool and milk samples linked to relevant clinical data including demographics, treatment and outcomes. Data has been presented and analysed by day of life, gestation at birth and CGA, all of which impact on this complex issue. The small number of exclusively formula milk fed infants reflects our comprehensive focus on preterm infants receiving MOM.
Although early milk samples were available, these remain limited, an inevitability as the focus for small volumes of early colostrum is administration to the baby, often directly from syringe rather than via feeding tubes and thus with no residual to sample. Milk samples used in this analysis carry information only on the day milk was fed to the infant, not when it was expressed. Whilst it is always true that a sample received on day 0–7 must have been expressed within this window, samples received by a baby later in life may have been expressed and stored from any point earlier. We now document both the day expressed and the day received by baby. Analyses have been presented by both DOL, birth gestation and CGA, but are not ‘adjusted’ to account for the relative impact of DOL on CGA at different CGA’s. For example, for a CGA of 23 weeks, given that survival before this is rare, any sample with a CGA of 23 weeks will by definition be a day 0–7 sample. However, at a CGA of 29 weeks samples will be a mixture of 29 week gestation infants on day 0 to 7, and more immature infants between 8 and 42 days old. Without much greater numbers of infants allowing separate analysis of each gestational cohort over time this challenge in interpretation and analysis will persist.
Small numbers of infants received no MOM and therefore were only ever fed formula (6 from an entire cohort of over 900 babies), meaning small number of infants contribute data pertaining to endogenous production. These six babies do not include the most preterm (median GA 28.7 (Range 26.2–31.7). Sampling from multiple units to capture these unusual babies would be necessary to improve numbers of exclusively formula fed babies in future studies. In a small number of samples from these formula milk fed infants we detected small quantities of IgA in the stool sooner than in overall: we hypothesise that this may result from buccal colostrum administration, but never receiving enough MOM enterally to ever be recorded as receiving MOM.
We have not included any attempt to quantify volumes of MOM fed to the infant on any day, which may be important, given that physiologically it is the total (s)IgA that is received that matters to the infant. Although sIgA concentration decreases over time, this corresponds to generally increasing volumes of milk received by the infant, and this may mean that the amount of sIgA interacting with the infant gut is relatively stable over time. In addition at the time of this study, we did not have access to donor milk, and therefore we have also not explored IgA in donor milk.
IgA in stool may exist bound to bacteria and as such concentration may be underestimated by ELISA, and this may account for some of the differences seen in the MOM-infant stool dyad IgA concentration.
Even colostrum produced after preterm delivery as early as 23 weeks contains high levels of IgA making this an important source of immunomodulation for preterm infants. The lack of endogenous sIgA production in the preterm gut until at least the 3 week of life means infants are dependent on exogenous supply until then. Until endogenous production is underway stool levels correlate with MOM levels. Whilst MOM is likely to be the best source, the potential role of donor milk and milk volumes received require further exploration.
The demonstration that IgA and sIgA concentrations are reduced through refrigeration, freezing and feeding practices is clinically important. The main function of sIgA in the intestinal lumen is to bind bacteria and either excrete pathogens or translocate commensal organisms across the intestinal epithelial layer, and if less sIgA available it would be logical that less IgA binding occurs. Mechanisms of this are not currently understood: IgA/sIgA may be being degraded by temperature changes or may bind to plastic and IgA may be more vulnerable to these processes than other immunoglobulins due to the higher content and glycosylation of IgA7. Future studies should explore these aspects further in order to identify handling practices in NICU that preserve maximal IgA levels. We have not explored all other potential factors that may impact on endogenous production of sIgA in the infant, nor have we extensive maternal information (for example data on body mass index, diet, maternal drugs etc) that may be impacting on MOM IgA levels, which could be done in future studies.
Given the data suggesting that IgA binding to gut bacteria protect against NEC, maximising exogenous IgA delivery until both endogenous production is established and the risk of NEC is low (e.g. after 32–33 weeks CGA22) is sensible. Exogenous non-MOM oral IgA has not been shown to be of benefit in reducing NEC, but data are limited. A Cochrane review in 2016 found that administration of oral immunoglobulins (IgG or a combination of IgG and IgA) did not reduce NEC incidence, need for surgical intervention or NEC-related death.29 The three included trials were heterogenous in nature, had high withdrawal rates and incomplete outcome data. Administration of sIgA rather than IgA, theoretically of the greatest benefit, has not been attempted.
Conclusions
These findings have clinical and translational relevance as sIgA has been shown to be important for binding potentially pathogenic bacteria in the preterm infant gut. In addition to ensuring all infants receive MOM, these data demonstrate the particular importance of successful colostrum expression and infant receipt, and the potential to target delivery of high concentration sIgA breast milk to the most preterm infants until a postnatal age where endogenous production increases. This is of particular relevance to preterm infants at risk of NEC.
In addition, research should include storage and handling/feeding practices that maximise sIgA available to the infant with potential beneficial impact.
References
Corpeleijn, W. E. et al. “Intake of own Mother’s milk during the first days of life is associated with decreased morbidity and mortality in very low birth weight infants during the first 60 days of life,”. Neonatology 102(Nov), 276–281 (2012).
Granger, C. L. et al. “Maternal breastmilk, infant gut microbiome and the impact on preterm infant health,”. Acta Paediatr. 110(Feb), 450–457 (2021).
Palmeira, P. & Carneiro-Sampaio, M. “Immunology of breast milk,”. Res Assoc. Med Bras. 62, 584–593 (2016).
Ballard, O. & Morrow, A. L. “Human Milk Composition: Nutrients and Bioactive Factors,”. Pediatr. Clin. North Am. 60, 49–74 (2012).
Mantis, N. J., Rol, N. & Corthesy, B. “Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut,”. Mucosal Immunol. 4, 603–611 (2011).
Relman, D. A. “Maternal IgA: matchmaking in early childhood,”. Immunity 51(Aug), 211–213 (2019).
Mantis, N. J. “Rediscovering IgA,” Mucosal Immunol., 588, 2011, https://doi.org/10.1038/mi.2011.42.
Demers-Mathieu, V., Underwood, M. A., Beverly, R. L., Nielsen, S. D. and Dallas, D. C. “Comparison of Human Milk Immunoglobulin Survival during Gastric Digestion between Preterm and Term Infants,” Nutrients 10, (2018). https://doi.org/10.3390/nu10050631.
Riskin, A. “Immunomodulatory Constituents of Human Donor Milk,”. Breastfeed. Med. 15(Sep), 563–567 (2020).
Bakker-Zierikzee, A. M. et al. “Faecal SIgA secretion in infants fed on pre- or probiotic infant formula,”. Pediatr. Allergy Immunol. 17(Mar), 134–140 (2006).
Dunne-Castagna, V. P. & Taft, D. H. “Mother’s Touch: Milk IgA and Protection from Necrotizing Enterocolitis,”. Cell Host Microbe 26(Aug), 147–148 (2019).
Gopalakrishna, K. P. and Hand, T. W. “Influence of maternal milk on the neonatal intestinal microbiome,” Nutrients 12. MDPI AG, Mar, (2020). https://doi.org/10.3390/nu12030823.
Trend, S. et al. “Levels of innate immune factors in preterm and term mothers’ breast milk during the 1st month postpartum,”. Br. J. Nutr. 115(Feb), 1178–1193 (2016).
Chandra, R. K. “Immunoglobulin and protein levels in breast milk produced by mothers of preterm infants,”. Nutr. Res. 2(Jan), 27–30 (1982).
Hsu, Y. C. et al. “Changes in preterm breast milk nutrient content in the first month,” Pediatr. Neonatol. (2014). https://doi.org/10.1016/j.pedneo.2014.03.002.
Ballabio, C. et al. “Immunoglobulin-a profile in breast milk from mothers delivering full term and preterm infants,” Int. J. Immunopathol. Pharmacol. 20, 119–128 (2007).
Velonà, T. et al. “Protein Profiles in Breast Milk from Mothers Delivering Term and Preterm Babies,”. Pediatr. Res. 45, 658–663 (1999). 1999 455.
Gross, S. J. et al. “Elevated IgA concentration in milk produced by mothers delivered of preterm infants,”. J. Pediatr. 99, 389–393 (1981).
Akhter, H., Aziz, F., Ullah, F. R., Ahsan, M. & Islam, S. N. “Immunoglobulins content in colostrum, transitional and mature milk of Bangladeshi mothers: Influence of parity and sociodemographic characteristics,”. J. Mother Child 24, 8–15 (2021).
Ogra, S. S. & Ogra, P. L. “Immunologic aspects of human colostrum and milk: II. Characteristics of lymphocyte reactivity and distribution of E-rosette forming cells at different times after the onset of lactation,”. J. Pediatr. 92(Apr), 550–555 (1978).
Demers-Mathieu, V. et al., “Differences in maternal immunoglobulins within mother’s own breast milk and donor breast milk and across digestion in preterm infants,” Nutrients 11, Apr. (2019). https://doi.org/10.3390/nu11040920.
Weaver, L. T., Arthur, H. M. L., Bunn, J. E. G. & Thomas, J. E. “Human milk IgA concentrations during the first year of lactation,”. Arch. Dis. Child 78, 235–239 (1998).
Gopalakrishna, K. P. et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat. Med. 25, 1110–1115 (2019).
Rognum, T. O., Thrane, P. S., Stoltenberg, L., Vege, A. & Brandtzaeg, P. “Development of Intestinal Mucosal Immunity in Fetal Life and the First Postnatal Months,”. Pediatr. Res 32, 145–149 (1992).
Gustafson, C. E. et al. Limited expression of APRIL and its receptors prior to intestinal IgA plasma cell development during human infancy. Mucosal Immunol. 7(Sep), 467–477 (2014).
Haworth, J. C. & Dilling, L. “Concentration of gamma-A-globulin in serum, saliva, and naso- pharyngeal secretions of infants and children,”. J. Lab Clin. Med 67, 922–933 (1966).
Rogier, E. W. et al. “Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression,”. Proc. Natl Acad. Sci. U. S. A. 111(Feb), 3074–3079 (2014).
Eibl, M. M., Wolf, H. M., Furnkranz, H. and Rosenkranz, A. “Prophylaxis of Necrotizing Enterocolitis by Oral IgA-IgG: Review of a Clinical Study in Low Birth Weight Infants and Discussion of the Pathogenic Role of Infection.” J. Clin. Immunol. 10, 725–795 (2004).
Foster, J. P., Cole, M. J., and Seth, R. “Oral immunoglobulin for preventing necrotizing enterocolitis in preterm and low birth weight neonates,” Coch. Database Syst. Rev. 2016, John Wiley and Sons Ltd, Apr, 2016, https://doi.org/10.1002/14651858.CD001816.pub3.
Funding
N.D.E. and J.E.B. declare institutional research funding from Prolacta Biosciences and Danone Early Life Nutrition, and honoraria from Danone Early Life Nutrition, and Nestle Nutrition Institute. C.J.S. declares receiving lecture honoraria from Danone Early Life Nutrition and Nestle Nutrition Institute, but has no share options or other conflicts. C..LG. has obtained a grant from the Human Milk Foundation (HMF), which partly funded this research.
Author information
Authors and Affiliations
Contributions
C.L.G., C.A.L., N.D.E., J.M.P., C.J.S. and J.E.B. were responsible for the study concept, design and methodology. Wet lab work was performed by C.L.G. supported by J.M.P. Data analysis and interpretation was led by C.L.G. supported by all authors. C.L.G. and J.E.B. drafted the paper with subsequent critical revisions and edits from all authors. All authors approved the final paper for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
Participants were recruited to an ongoing REC approved sample salvage study (SERVIS Supporting Research in Vulnerable Infants REC 10/H0908/39). No additional consent was required for this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Granger, C.L., Lamb, C.A., Embleton, N.D. et al. Secretory immunoglobulin A in preterm infants: determination of normal values in breast milk and stool. Pediatr Res 92, 979–986 (2022). https://doi.org/10.1038/s41390-021-01930-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01930-8
- Springer Nature America, Inc.