Abstract
Metal–organic frameworks (MOFs) are a group of porous materials that display potential in the elimination of toxic industrial compounds (TICs) from polluted water streams. However, their applications have so far been held up by issues due to their physical nature and cost. In this study, activated carbon (AC) is modified with an Fe-based MOF, iron terephthalate (Fe-BDC). A facile and cost-effective impregnation method is used for enhanced removal from aqueous solutions. The new adsorbent is characterized by SEM, FTIR, PXRD, and BET. The composite displays excellent uptake of Cr (VI) when compared to un-impregnated AC with a maximum monolayer adsorption capacity of 100 mg·g−1. The experimental data shows a high correlation to the Langmuir adsorption model. The adsorption kinetic study reveals that the adsorption of Cr (VI) to Fe-BDC@AC obeys the pseudo-first-order equation. The composite shows high reusability after five cycles and high adsorption rates reaching equilibrium in just 50 min. Such properties make the nanocomposite promising for water decontamination on larger scales compared to powder-based alternatives, such as individual MOF crystals.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Chromium(VI) is a hazardous contaminant categorized as a group ‘A’ human carcinogen by the U.S. Environmental Protection Agency (EPA) [1]. Chromium (Cr) concentrations in drinking water are limited to 0.1 ppm by the EPA [2,3,4]. Cr is considered to be one of the most toxic inorganic pollutants due to its carcinogenic effects on biological species [5]. Chromium is widely used in many industries and applications resulting in large quantities of hazardous waste. One application is hardening steel and manufacturing stainless steel, which is an essential raw material for construction, heavy machinery, automobiles, transportation, energy, and medical industries [6]. Chromium is also widely used in chrome plating processes, paint pigments, and the production of dyes, leather, plastics, and photographs. In an aqueous environment, chromium usually exists as trivalent Cr(III) or hexavalent Cr (VI) oxidation state. Ionic substances containing Cr (VI), such as CrO42− or HCrO4−, have higher solubility and fluidity than Cr(III) in nature, so substances containing Cr (VI) can more easily pass through cell membranes than Cr(III) [7]. It is estimated that in an aqueous environment, Cr (VI) is almost 100 times more toxic than Cr(III) [8]. Contaminants associated with the continental crust typically have a maximum Cr concentration of 100 ppm, but many studies test at high concentrations which may inflate adsorption capacity [9].
Many methods such as adsorption [10,11,12], ion exchange [13], membrane separation [14], coagulation [15], chemical precipitation [16], extraction [17], and electrochemical separation [18] have been proven to remove organic contaminants and heavy metal ions, such as hexavalent chromium [19], from sewage. Among these different methods, adsorption may be the most efficient, economically feasible, environmentally sustainable, and technologically promising process [20, 21]. Recently, various adsorbents such as activated carbon [20, 22], layered double hydroxides (LDHs) [23], polymers and biomass-based materials [24] have been used to remove Cr (VI). In general, these adsorbents suffer from low and slow Cr (VI) sorption, and limited selectivity [25, 26].
Metal–organic frameworks (MOFs) materials have been proven to be very effective in adsorbing Cr (VI), and their highly branched structures can be easily functionalized to selectively adsorb and remove different heavy metal ions [27,28,29,30]. One of the key issues concerning the potential usage of MOFs in wastewater treatment is their physical form. MOFs normally form fine powders, which hinder their application in batch and continuous adsorption processes [31]. Form-changing processes such as pelletization have been explored to overcome this problem. However, these processes increase engineering and synthetic costs beyond cheaper adsorptive materials such as activated carbon [32]. One alternative may be to merge a MOF and activated carbon into a porous composite material that displays positive features from both materials.
In this study, the MOF selected is MIL-53 Fe (Fe-BDC). The impregnation of Fe-BDC within the AC enhances the adsorption efficiency of active carbon towards Cr (VI). Fe-BDC is chosen because Fe acts as an electron donor to form less toxic and less water soluble states such as Cr (III) [33]. The higher electron density and lower water solubility make the material easier to remove by chemisorption or physisorption with AC. Fe is also comparably lower cost than other metals, such as copper.
This material has been used in the photocatalytic removal of organic contaminants [34]. One limitation is that pristine Fe-BDC does not have a high surface area compared to most MOFs. However, Fe-BDC can open its pores only in the presence of guest particles owing to its breathing feature and flexible structure [35]. There are a limited number of studies on MOFs-activated carbon nanocomposites used for Cr (VI) sorption. In this study, we synthesize and characterize a novel Fe-BDC@AC nanocomposite. Subsequently, we test the adsorption interaction between Fe-BDC@AC and Cr (VI) ion in an aqueous solution and study the adsorption mechanism and characteristics of Cr (VI) ion onto Fe-BDC@AC.
2 Experimental Procedure
2.1 Materials
Cinnamon sticks were obtained from the local market, as they are widely used in Egypt. Iron chloride (FeCl3.6H2O, 98%, Sigma Aldrich), 1,4 benzene dicarboxylic acid (BDC, 98%, Sigma Aldrich), acetic acid (98%, Sigma Aldrich), and N, N-dimethylformamide (DMF, 98%, Sigma Aldrich), are used for the synthesis of Fe-BDC. HCl (37%, Alfa Aesar) was used for regeneration purposes. Absolute ethanol, acetone, and deionized water (DIW, all from Alfa Aesar) are used for washing purposes.
2.2 Synthesis of Fe-BDC
Samples of Fe-BDC are synthesized through sequential addition of FeCl3·6H2O (0.27 g, 1 mmol) 1,4-benzene dicarboxylic acid (BDC) (0.166 g, mmol), and acetic acid (1 mL) into a beaker. Afterwards, 20 mL of N, N-dimethylformamide (DMF) is added to completely dissolve all reagents [36]. The mixture is poured into a closed vial and placed in an oven at 100 °C for 6 h. The resulting powder, Fe-BDC, is recovered by centrifugation at 6000 rpm for 30 min and rinsed rigorously three times with each of DMF, ethanol, acetone, and water. Finally, the recovered crystals are placed into an oven set at 140 °C to dry overnight.
2.3 Preparation of Cinnamon Sticks-Based Activated Carbon
The obtained raw materials (Cinnamon sticks) were washed with distilled water to remove the contaminants after dissolving the undesirable substances, dehydrated at 110 °C for 24 h., grounded, and sieved to achieve particle sizes below 2 mm in diameter. The dehydrated cinnamon granules were pyrolyzed to 900 °C, with a heating rate of 10 °C/min and a dwell time of 2 h under constant nitrogen gas flowing at 150 cm3/min. The activation process was done by using carbon dioxide as an activating agent. The carbonized samples were activated at 900 °C with a holding time of 120 min. The heating rate was 20 °C/min, and the flow rate of CO2 was 150 cm3/min. Additional details about the fabrication process of AC follow previous work [37].
2.4 Synthesis of Fe-BDC@AC
For the preparation of Fe-BDC@AC, a mixture of terephthalic acid (0.166 g, 1 mmol) and AC (150 mg) is mixed and sonicated in 10 mL DMF for 5 min. A separately prepared solution of FeCl3·6H2O (0.27 g, 1 mmol) in 5 mL DMF and 1 mL acetic acid is then added. The vial is capped and the mixture is stirred for 8 h at 100 °C. The product is filtered and then dried in an isothermal oven at 120 °C overnight.
2.5 Characterization and Instrumentation
The synthesized Fe-BDC and Fe-BDC@AC are characterized using several techniques. Fourier transform infrared spectroscopy (FTIR, Jasco FT/IR 4100) using a KBr pellet method and Raman (Bruker Sentera) are used to identify the chemical structure. Powder X-ray diffraction (PXRD, Shimadzu XD-l) is used to verify the crystalline nature. SEM/EDX (Zeiss EVO-10 microscopy) are used to determine surface morphology and elemental composition, respectively. The X-ray photoelectron spectrum (XPS, Thermo Fisher Scientific K-ALPHA) was acquired with a monochromatic Al-Kα (1486.7 eV). Nitrogen adsorption tests (NOVA Station A) are used to calculate BET surface areas.
2.6 Adsorption Studies
Adsorption experiments are accomplished in a set of 150 mL Erlenmeyer conical flasks filled with 50 mL solutions of DIW and different initial concentrations of Cr (VI) (5–50 mg/L). The solution's initial pH was adjusted to ≈ 5.5 by (0.1 M) HCl and/or NaOH. The solution was continually stirred by an orbital shaker (SI-300R). 50 mg samples of impregnated active carbon (Fe-BDC@AC) with mesh size (18*30) are used in each adsorption study. Treated solutions of Cr (VI) samples are regularly withdrawn every 5-min to measure the concentration with a double beam UV–Vis spectrophotometer (Shimadzu, Japan). A wavelength of 375 nm (λmax) is implemented for all absorbance measurements. Eq. (1) is utilized to calculate the Cr (VI) removal efficiency (%R) where C0 and Ct (mg·L−1) indicate the initial Cr (VI) concentration and the concentration at time interval t.
All experiments are conducted in temperature-controlled environments at 25 °C. The adsorption capacities at equilibrium (qe, mg·g−1) are measured by applying Eq. (2) where Ce (mg·L−1) implies the concentration after equilibrium for the Cr (VI) solutions, w denotes the mass of the adsorbent (g), and V is the volume of Cr (VI) solution.
3 Results and Discussion
3.1 Characterization
Freshly prepared Fe-BDC and AC, as well as the nanocomposite Fe-BDC@AC, are characterized using SEM, PXRD, FTIR, and BET. The SEM image of AC, as seen in Fig. 1a, shows its highly porous nature. Figure 1b shows the SEM images of Fe-BDC with a diamond-like crystalline structure, similar to previous literature reports [38, 39]. This confirms the successful synthesis of Fe-BDC. The nanocomposite elemental mapping is shown in Fig. 1(c, d, e, f) where Fe-BDC covers the outer surface and macropores of the AC. This confirms the successful fabrication of Fe-BDC@AC.
The XRD patterns of Fe-BDC and Fe-BDC@AC are shown in Fig. 2a. The recorded 2θ values are consistent with the single crystal simulation data reported in literature [40]. The primary peaks are identified at 2θ = 9.4°, 12.6°, 16.4°, 18.8°, 19.5°, 22.0° and 28.5°, corresponding to (002), (101), (102), (103), (200), (202) and (211) planes, respectively[41]. Substantial alterations in the peaks can be noted between Fe-BDC and the functionalized Fe-BDC@AC. A broad C peak corresponding to the (002) plane can be observed centred at 23.3°. Another carbon peak can also be observed at 43.1° corresponding to the (101) plane.
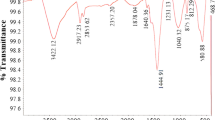
FTIR spectra of AC and Fe-BDC@AC are shown in Fig. 2b. Both show two strong peaks at 1377 and 1585 cm–1 that are associated with the characteristic antisymmetric and symmetric O–C = O stretching frequency vibrations, respectively. AC shows a weak peak at 873 cm−1 corresponding to C-H vibrations on aromatic rings that are shifted to 748 cm–1 in the composite, as a result of the newly introduced benzene rings. By comparison, the Fe-BDC@AC sample exhibits a broad peak at 3422 cm–1 attributed to the O–H stretching mode of the benzene ring in the BDC molecule. The metal–oxygen bond vibration (ν[M–O]) at 543 cm–1 is attributed to the interactions between the carboxyl group of terephthalic acid and Fe atoms [42]. The specific surface area of the AC decreased from 780 to 496 m2/g when FE-BDC is loaded onto the AC. Figure 2c shows the substantial reduction in N2 adsorption as a result of Fe-BDC covering the surfaces and filling the pores of the AC. However, the impregnation of Fe-BDC within the pores of AC greatly enhances the specific adsorption of Cr (VI) due to the presence of FeO6 metal centers [36].
Figure 3a,b represents the XPS spectra of Fe-BDC@AC before and after the adsorption of Cr (VI). The presence of the principal Fe2p, C1s, and O1s core levels before and after adsorption with relatively similar abundances suggest good chemical stability of Fe-BDC@AC during the adsorption process. Moreover, residual chlorine was observed before adsorption tests from the precursor FeCl3. It was completely detached and substituted with chromium after adsorption tests with Cr (VI).
The Cl2p core-level spectral envelope was adequately decomposed into three spin–orbit split doublets (Cl2p3/2 and Cl2p1/2) as shown in Fig. 3c, indicating three different electronic environments of chorine. The binding energy values for the Cl2p3/2 peaks resided at 197.7, 199.0, and 200.1 eV. The first and last components suggest the presence of the ion (Cl¯) [43,44,45] and covalent (–Cl) [43, 45,46,47,48] chlorine species, respectively. The chlorine species with intermediate binding energy (Cl*) is more appropriately associated with charge-transfer interactions between the coordinated chlorine and metal [49,50,51,52]. Further, this binding energy contribution may also be referred to as the free Cl¯ ion [53]. The various Cl components were expected since Cl is mainly bonded/coordinated with metallic Fe. Interestingly, all chlorine species have completely vanished after successive adsorption processes of Cr (VI). The newly observed Cr2p peaks (Fig. 3d) at 577.78, 582.31, 579.65, and 588.09 eV are attributed to Cr(III) [54,55,56,57,58,59,60] and Cr (VI) species [57,58,59,60,61], respectively. Cr (VI) was instantaneously adsorbed onto the surfaces of the Fe-BDC@AC by electrostatic attraction and ion exchange due to the positively charged surfaces, hydroxyl, and ironoxy groups of the Fe-BDC@AC. The Cr(III) could appear due to Cr (VI) being reduced by the AC directly [62]. Alternatively, FeO6 metal cluster nodes could be acting as redox mediators to enhance the reduction of Cr (VI) to Cr (III) [63].
3.2 Effect of Contact Time and Initial Cr (VI) Concentration on the Adsorption Capacities of (Fe-BDC@AC) at Equilibrium
The effect of contact time on Cr (VI) adsorption is investigated at a concentration of 25 mg/L by measuring the decrease in absorbance at a wavelength of 375 nm (λmax). The results are shown in Fig. 4a. All the experiments are conducted at static conditions of carbon dose (50 mg), pH (5.5), room temperature (25 ± 1 ºC), and 100 rpm agitation speed. The absorbance of Cr (VI) at a wavelength of 375 nm (λmax) decreases with an increase in contact time, indicating an increase of adsorption capacity and percent removal. No noticeable change in adsorption capacity or percent removal can be observed between 50 and 55 min, suggesting saturation of the material. The percent removal after 55 min was 85%. Therefore, the optimum contact time is approximately 55 min. This is also the equilibrium time of the batch adsorption experiments because adsorption is not changed beyond a contact time of 55 min. Equilibrium is attributed to an increase in the accumulation of Cr (VI) and the saturation of active sites and pores. The AC alone shows substantially lower adsorption capabilities, as shown in Fig. 4a. Therefore, the primary adsorption mechanism is hypothesized to be the breathing mechanism of the Fe-BDC. However, the powder-like nature of MOFs such as Fe-BDC makes it unfeasible for large-scale application in water decontamination. Secondly, the adsorption of Cr (VI) by (Fe-BDC@AC) is studied at different initial concentrations of 5,10, 15, and 25 ppm.
(a) Effect of initial Cr (VI) concentration on the equilibrium adsorption capacity; (b) removal percentages at different initial Cr (VI) concentrations; (c) effects of different Fe-BDC@AC doses on removal percentage for Cr (VI) at 5 ppm; (d) removal efficiency of Fe-BDC@AC against organic dyes at 5 ppm. All tests are conducted with 50 mg of adsorbent, a pH of 5.5 and room temperature (25 ± 1 ºC) conditions
The final percentage of Cr (VI) removed decreases as the initial concentration increases, as shown in Fig. 4b. A relatively low removal efficiency of 61% can be observed at 25 ppm owing to the limited pore volume and surface area of the nanocomposite. Fe-BDC@AC doses (25–200 mg) have also been studied against 5 ppm of Cr (VI) concentrations. As expected, the removal percentages have been increased with increasing absorbents doses, as shown in Fig. 4c. More active sites and vacancies are available with increasing the doses of Fe-BDC@AC, resulting in higher adsorption capacity and removal percentages.
The removal efficiency of the nanocomposite was checked against two different dyes: negatively charged methyl orange and the positively charged methylene blue, as shown in Fig. 4d. The removal efficiency has been dramatically decreased with methylene blue dye which may be due to the positively charged open metal center in the Fe-BDC@AC [64]. The removal efficiency of methyl orange is much higher than methylene blue likely due to the negative charge comparable to Cr(VI). However, the molecular size of methyl orange (1.2 nm) is higher than the dichromate ion (calculated as 0.65 nm) [65]. The larger molecular size limits physical adsorption leading to lower removal efficiency than Cr(VI).
According to the classification of adsorption isotherms in an aqueous phase, the adsorption of Cr (VI) by the prepared (Fe-BDC@AC) sample is classified as class L (Langmuir-type) [66]. This type of isotherm refers to circumstances when there is no strong competition on the quantity of the solvent molecules in the coverage of the active sites on the adsorbent surface (Fe-BDC@AC) by the adsorbate (Cr (VI)). Here the adsorbate molecules are arranged horizontally in the adsorption layer [66]. Maximum sorption capacity was observed at 25 ppm. The influence of the initial Cr (VI) concentration is governed by the immediate relation between the Cr (VI) concentration and the available active binding sites on an adsorbent surface [67]. Commonly, the percent of Cr (VI) removal decreases with an increase in initial concentration, which may be due to the complete occupation of adsorption sites on the adsorbent exterior [68]. At low Cr (VI) concentrations, there will be empty and available active binding sites on the (Fe-BDC@AC). When the initial Cr (VI) concentration increases, the active sites needed for adsorption of the Cr (VI) molecules will close [69]. Still, an increase in Cr (VI) concentration will increase the adsorption capacity. This may be due to the excessive driving forces as a result of excess mass or chemical potential at higher initial Cr (VI) concentrations [70]. At lower concentrations, the ratio between Cr (VI) molecules to the free adsorption sites is small. Consequently, the adsorption shifts are independent of the original concentration. However, the available sites of adsorption decrease at high concentrations. Hence the percent removal of Cr (VI) is dependent upon initial concentration.
3.3 Adsorption Equilibrium Isotherms
Three isotherms were verified for their capability to define experimental results, namely the Langmuir, Freundlich, and the Temkin isotherm. Equation (3) expresses the Langmuir model where qe (mg·g−1) and Ce (mg·L−1) is Cr (VI) adsorbed per unit mass of Fe-BDC@AC and Cr (VI) concentration at equilibrium, respectively. The maximum quantity of the Cr (VI) per unit mass of Fe-BDC@AC to form a whole monolayer on the surface-bound at high Ce is qmax. The constant KL is referred to as the affinity of the binding sites (L·mg−1).
The outline of specific adsorption (Ce/qe) against the steadiness concentration (Ce) illustrates that the adsorption conforms to the Langmuir model, as shown in Fig. 5a. The Langmuir constants qmax and KL are determined from the plot's slope and intercept, respectively. The results are tabulated in Table 1. The separation factor, RL, is a critical characteristics of the Langmuir isotherm calculated by Eq. (4). C0 is the uppermost initial Cr (VI) concentration (mg·L−1), and KL (L·mg−1) is the Langmuir constant.
The adsorption is modelled by (a) Langmuir, (b) Freundlich, and (c) Temkin isotherms for the removal of Cr (VI) by Fe-BDC@AC. The kinetics are modelled by (d) pseudo-first-order and (e) pseudo-second-order. Reusability tests showing the ability of the Fe-BDC@AC to maintain removal efficiency for 5 ppm Cr (VI) concentration is shown in (f)
The RL value determines if the isotherm is unfavorable (RL > 1), linear (RL = 1), irreversible (RL = 0), or favorable (0 < RL < 1). The experimental value of RL is found to be 0.10 which demonstrates that the adsorption of Cr (VI) on Fe-BDC@AC is favourable. In addition, the Fe-BDC@AC sample shows excellent hexavalent chromium mono-molecular uptake of 100 mg·g−1 compared to other adsorbents available in literature [10, 15, 24, 25], as shown in Table 2. Activated carbon in this work is primarily used as high porosity solid support for Fe-BDC to enable enhanced Cr (VI) adsorption efficiency in industrial applications and increase the sorbent–solute contact time compared to non-impregnated samples which show lower adsorption potential to Cr (VI).
Equation (5) is used to describe a heterogeneous system by the Freundlich isotherm [5]. The Freundlich coefficient, KF (mg·g−1·L·mg−1·n−1), is the sorbent's adsorption capacity, and n represents whether adsorption is favourable. Values of n > 1 signify favourable adsorption. Values of n and KF are calculated from the slope and intercept of Fig. 5b. The results are recorded in Table 1.
The Temkin isotherm is extended to determine whether it is physical or chemical sorption, as shown in Eq. (6). It has commonly been applied in the linear form as described in Eq. (6), where b is a constant linked to the sorption heat (J·mol−1), KT is the Temkin isotherm constant (L·g−1), R is the gas constant (8.314 J·mol−1·K−1), and T is the absolute temperature (K). The constants KT and b can be estimated by plotting qe versus ln(Ce) in Fig. 5c. If the sorption heat is less than 1.0 kcal·mol−1, then physical adsorption occurs. If the value is between 20 and 50 kcal/mol, then chemical adsorption is occurring. If the sorption value is between 1.0 and 20 kcal/mol, then both chemical and physical adsorption are occurring. As seen in Table 1, the Langmuir isotherm matches quite well with the experimental data, as can be seen from a correlation coefficient R2 > 0.99. From the Langmuir adsorption model, the monolayer adsorption capacity is approximated as 100 mg·g−1. This value is slightly higher than values observed experimentally (~ 90 mg·g−1) but is within reasonable range if the initial Cr (VI) concentration or adsorption time is further increased. Fe-BDC active sites are assumed to have homogeneous distribution within the Fe-BDC@AC nanocomposite because the Langmuir equation assumes that the surface is similar or homogenous.
3.4 Adsorption Kinetics
Kinetic adsorption outcomes for different Cr (VI) concentrations were examined using a pseudo-first-order model and a pseudo-second-order model. For the pseudo-first-order kinetic model, k1 values are obtained from the slopes of the linear log plots (qe–qt) versus t, as shown in Fig. 5d. The correlation coefficient value is determined to be 0.990. The experimental qe values are well-aligned with the estimated values obtained from the linear plots, as seen in Table 3. This shows that the adsorption of Cr (VI) to Fe-BDC@AC obeys the pseudo-first-order equation. A linear plot of t/qt versus t is generated for the pseudo-second-order kinetic model, as illustrated in Fig. 5e. A weak relationship between the experimental and measured qe values can be observed, as verified in Table 3. Moreover, the correlation coefficient for the second-order kinetic model is only 0.85 for the adsorption of Cr (VI). This indicates the second-order kinetic model is less suitable to explain the Cr (VI) adsorption mechanism on the prepared Fe-BDC@AC sample.
The adsorption of Cr (VI) occurs relatively rapidly in our experiments. As can be seen from Table 2, the synthesized Fe-BDC@AC reaches max adsorption uptake or adsorption equilibrium between 50 and 55 min. By comparison, many literature values and commercially activated carbons take approximately three times this duration to reach adsorption equilibrium.
For the possible assessment of the performance of adsorbent systems, regeneration and reusability are important influencing factors. The desorption of Cr (VI) could be achieved by using a solution of 1 M HCl in the system to allow for ions exchange. Reusability experiments are conducted for five adsorption/desorption cycles. The percent removal of 5 ppm Cr (VI) is plotted in Fig. 5f. The results show that the removal efficiency decreases slightly from 85 to 80%. This confirms that the Fe-BDC@AC can retain its efficiency to an acceptable extent, and can be reused effectively. This shows the promise of MOF-AC nanocomposites for commercial applications where both adsorption rate and reusability are extremely important parameters.
3.5 Adsorption Mechanism of Cr (VI)
The results showed that Langmuir isotherm matches well with the experimental data (correlation coefficient R2 > 0.99). The adsorption kinetic study reveals that the adsorption of Cr (VI) to Fe-BDC@AC obeys the pseudo-first-order equation. These results suggest that the uptake of Cr (VI) is most likely due to physical adsorption. Therefore, the surface area is one important characteristic of the adsorbent. However, the adsorption capacity increases substantially after impregnating with Fe-BDC despite substantially reducing the BET surface area. Therefore, surface properties must play a larger role in the physical adsorption process. Electrostatic attraction between the positive surface charge of the adsorbents (AC) due to its impregnation with Fe-BDC and negatively charged Cr (VI) is critical to the mechanism, as indicated in Fig. 6. A similar trend is reported on methyl orange sorption on zirconium-based MOF [75] and chitosan/nanodiamond composite [76]. There have been previous studies that report carbonaceous adsorbents act as electron donors to reduce Cr (VI) to Cr(III)[77]. Furthermore, the presence of Cl within the MOF act as an avenue for ion exchange with the heavy metal Cr (VI)[78]. It is expected that these mechanisms further enhance the adsorption kinetics and increase the favourability of physical adsorption.
4 Conclusions
In conclusion, this study shows the synthesis of MOF-carbon nanocomposite materials. The facile functionalization of Fe-BDC onto AC generated from waste cinnamon bark is a promising, low-cost material for Cr (VI) adsorption. SEM imaging shows that the Fe-BDC covers the outer surface and fills the macropores of the AC. The removal efficiency of the new composite for Cr (VI) reaches 85% in just 50 min which is a substantially shorter time than most sorbents, such as commercial AC. One limitation of this work is higher concentrations of Cr(VI). The removal efficiency is reduced to 61% at an initial concentration of 25 ppm, suggesting more than 0.05 g of adsorbent may be required. The adsorption of the Cr (VI) on Fe-BDC@AC adsorbents could be described by the pseudo-first-order kinetic and the Langmuir isotherm model. The adsorption capacity reached up to 100 mg·g−1 which is substantially higher than AC alone. The adsorption mechanism includes electrostatic attractions and ion exchange between negatively charged Cr (VI) and the positive surface charge of Fe and AC. The materials may be tailored to remove a broader range of contaminants than the individual components alone by controlling surface area, pore size and surface properties. This approach can be extended to the construction of other MOFs on activated carbon granules, as well as other solid supports with relative ease allowing potential application of powder-based MOFs.
Data availability
Not applicable.
References
R.M. Park et al., Hexavalent chromium and lung cancer in the chromate industry: a quantitative risk assessment. Risk Anal Int J 24(5), 1099–1108 (2004)
Zhitkovich, A.J.C.r.i.t., Chromium in drinking water: sources, metabolism, and cancer risks. 2011. 24(10): p. 1617–1629.
Organization, W.H., Chromium in Drinking-water. 2020, World Health Organization.
Smith, A.H. and C.M.J.A.r.o.p.h. Steinmaus, Health effects of arsenic and chromium in drinking water: recent human findings. 2009. 30: p. 107–122.
Ali, H.M., et al., Selective and efficient sequestration of Cr (VI) in ground water using trimethyloctadecylammonium bromide impregnated on Artemisia monosperma plant powder. Journal of the Taiwan Institute of Chemical Engineers, 2021.
ATSDR, U., Toxicological profile for chromium. In: US Department of Health and Human Services, Public Health Service, 2012.
A. Zhitkovich, Chromium in drinking water: sources, metabolism, and cancer risks. Chem. Res Toxicol 24(10), 1617–1629 (2011)
M. Megharaj, S. Avudainayagam, R. Naidu, Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste. Curr Microbiol 47(1), 0051–0054 (2003)
P Bonnand et al 2013 The chromium isotopic composition of seawater and marine carbonates. 382 10 20
N. Fellenz et al., Chromium (VI) removal from water by means of adsorption-reduction at the surface of amino-functionalized MCM-41 sorbents. Microporous Mesoporous Mater. 239, 138–146 (2017)
A.M., Aldawsari et al., Tailoring an efficient nanocomposite of activated carbon-layered double hydroxide for elimination of water-soluble dyes. J Alloy Comp 857, 157551 (2021)
A.M. Aldawsari et al., Activated carbon/MOFs composite: AC/NH2-MIL-101 (Cr), synthesis and application in high performance adsorption of p-nitrophenol. J Saudi Chem Soc 24(9), 693–703 (2020)
W.A. El-Mehalmey et al., Metal–organic framework@ silica as a stationary phase sorbent for rapid and cost-effective removal of hexavalent chromium. J Mat Chem A 6(6), 2742–2751 (2018)
C.A. Kozlowski, W. Walkowiak, Removal of chromium (VI) from aqueous solutions by polymer inclusion membranes. Water Res. 36(19), 4870–4876 (2002)
G., Lee, J.G., Hering, Removal of chromium (VI) from drinking water by redox-assisted coagulation with iron (II). J Water Supply Res Tech—AQUA 52(5), 319–332 (2003)
H. Peng et al., High-efficient recovery of chromium (VI) with lead sulfate. J Taiwan Inst Chem Eng 85, 149–154 (2018)
A. Agrawal, C. Pal, K. Sahu, Extractive removal of chromium (VI) from industrial waste solution. J Hazard Mater 159(2–3), 458–464 (2008)
P. Gao et al., Removal of chromium (VI) from wastewater by combined electrocoagulation–electroflotation without a filter. Sep Purif Technol 43(2), 117–123 (2005)
Adsorption of Cr (VI) using α-Fe2O3 coated hydroxy magnesium silicate (HMS): isotherm, thermodynamic and kinetic study. Int J Environ Anal Chem , 1–17 (2021). https://doi.org/10.1080/03067319.2021.1890061
M. Pérez-Candela, J. Martín-Martínez, R. Torregrosa-Maciá, Chromium (VI) removal with activated carbons. Water Res 29(9), 2174–2180 (1995)
A.M. Aldawsari et al., Adsorptive performance of aminoterephthalic acid modified oxidized activated carbon for malachite green dye: mechanism, kinetic and thermodynamic studies. Sep Sci Technol 56(5), 835–846 (2021)
M. Hasanzadeh, A. Simchi, H.S. Far, Nanoporous composites of activated carbon-metal organic frameworks for organic dye adsorption: Synthesis, adsorption mechanism and kinetics studies. J Ind Eng Chem 81, 405–414 (2020)
Nalawade, P., et al., Layered double hydroxides: A review. 2009.
E. Finocchio et al., Chromium (VI) removal by methylated biomass of Spirulina platensis: the effect of methylation process. Chem Eng J 156(2), 264–269 (2010)
Karthikeyan, T., S. Rajgopal, and L.R.J.J.o.h.m. Miranda, Chromium (VI) adsorption from aqueous solution by Hevea Brasilinesis sawdust activated carbon. 2005. 124(1–3): p. 192–199.
Islam, M.A., et al., Recent innovative research on chromium (VI) adsorption mechanism. 2019. 12: p. 100267.
L.-L. Li et al., Cr (VI) removal via anion exchange on a silver-triazolate MOF. J Hazard Mater 321, 622–628 (2017)
J., Guo, J.-J., Li, C.-C., Wang, Adsorptive removal of Cr (VI) from simulated wastewater in MOF BUC-17 ultrafine powder. J Environ Chem Eng 7(1), 102909 (2019)
H. Shahriyari Far et al., Efficient removal of Pb (II) and Co (II) ions from aqueous solution with a chromium-based metal-organic framework/activated carbon composites. Ind Eng Chem Res 60(11), 4332–4341 (2021)
O Abuzalat et al 2022 Nano-porous bimetallic organic frameworks (Fe/Co)-BDC, a breathing MOF for rapid and capacitive removal of Cr-oxyanions from water.J Water Proc Eng 46 102537.
Wang, Q. and D.J.C.r. Astruc, State of the art and prospects in metal–organic framework (MOF)-based and MOF-derived nanocatalysis. 2019. 120(2): p. 1438–1511.
Koh, K., A.G. Wong-Foy, and A.J.J.C.C. Matzger, MOF@ MOF: microporous core–shell architectures. 2009(41): p. 6162–6164.
WooáLee, J. and S.J.N. BináKim, Enhanced Cr (VI) removal using iron nanoparticle decorated graphene. 2011. 3(9): p. 3583–3585.
A.A. Oladipo, MIL-53 (Fe)-based photo-sensitive composite for degradation of organochlorinated herbicide and enhanced reduction of Cr (VI). Process Saf Environ Prot 116, 413–423 (2018)
A. Schneemann et al., Flexible metal–organic frameworks. Chem. Soc. Rev. 43(16), 6062–6096 (2014)
M. Su et al., Enhanced hexavalent chromium removal by activated carbon modified with micro-sized goethite using a facile impregnation method. Sci Total Environ 647, 47–56 (2019)
Ettish, M.N., et al., Preparation and characterization of new adsorbent from Cinnamon waste by physical activation for removal of Chlorpyrifos. Environmental Challenges, 2021: p. 100208.
M Knyazeva et al 2018 Synthesis and structural-energy characteristics of Fe-BDC metal-organic frameworks. 54 6 1004 1009
He, Q., et al., Facile fabrication of Fe-BDC/Fe-2MI heterojunction with boosted photocatalytic activity for Cr (VI) reduction. 2021. 9(5): p. 105961.
T.R. Whitfield et al., Metal-organic frameworks based on iron oxide octahedral chains connected by benzenedicarboxylate dianions. Solid State Sci 7(9), 1096–1103 (2005)
Rajpurohit, A.S., D.K. Bora, and A.K.J.A.M. Srivastava, Simultaneous determination of amlodipine and losartan using an iron metal–organic framework/mesoporous carbon nanocomposite-modified glassy carbon electrode by differential pulse voltammetry. 2018. 10(45): p. 5423–5438.
J. Gordon, H. Kazemian, S. Rohani, MIL-53 (Fe), MIL-101, and SBA-15 porous materials: potential platforms for drug delivery. Mater Sci Eng C 47, 172–179 (2015)
E.T. Kang et al., Structural studies of poly(p-phenyleneamine) and its oxidation. Macromolecules 23(11), 2918–2926 (1990)
E.T. Kang, K.G. Neoh, K.L. Tan, Polyaniline: A polymer with many interesting intrinsic redox states. Prog Polym Sci 23(2), 277–324 (1998)
A.P. Monkman, G.C. Stevens, D. Bloor, X-ray photoelectron spectroscopic investigations of the chain structure and doping mechanisms in polyaniline. J Phys D Appl Phys 24(5), 738–749 (1991)
M. Aldissi, S.P. Armes, X-ray photoelectron spectroscopy study of bulk and colloidal polyaniline. Macromolecules 25(11), 2963–2968 (1992)
K.L. Tan et al., X-ray photoelectron spectroscopy studies of the chemical structure of polyaniline. Phys Rev B Condens Matter 39(11), 8070–8073 (1989)
P. Snauwaert et al., A photoelectron spectroscopic study of the electrochemical processes in polyaniline. J Chem Phys 92, 2187 (1990)
S.A. Martynova et al., Exothermal effects in the thermal decomposition of [IrCl6]2−-containing salts with [M(NH3)5Cl]2+ cations: [M(NH3)5Cl][IrCl6] (M = Co, Cr, Ru, Rh, Ir). New J Chem 42(3), 1762–1770 (2018)
A.J. Pardey et al., Spectroscopic characterization of coordination complexes based on dichlorocopper(II) and poly(4-vinylpyridine): Application in catalysis. Polyhedron 24(4), 511–519 (2005)
D.P. Drolet et al., FT-IR and XPS study of copper(II) complexes of imidazole and benzimidazole. Inorg Chim Acta 146(2), 173–180 (1988)
M.E. Quiroga et al., 1-Heptyne semihydrogenation catalized by palladium or rhodium complexes: Influence of: metal atom, ligands and the homo/heterogeneous condition. Appl Catal A 326(2), 121–129 (2007)
J.P. Bruce, J.C. Hemminger, Characterization of Fe2+ aqueous solutions with liquid jet X-ray photoelectron spectroscopy: chloride depletion at the liquid/vapor interface due to complexation with Fe2+. J. Phys. Chem. B 123(39), 8285–8290 (2019)
R.S. Vieira et al., Copper, mercury and chromium adsorption on natural and crosslinked chitosan films: An XPS investigation of mechanism. Colloids Surf., A 374(1), 108–114 (2011)
A. Rahman et al., Characterization of chromia/alumina catalysts by X-ray photoelectron spectroscopy, proton induced X-ray emission and thermogravimetric analysis. Appl Catal A 121(2), 203–216 (1995)
P.G. Harrison, N.C. Lloyd, W. Daniell, The nature of the chromium species formed during the thermal activation of chromium-promoted tin (IV) oxide catalysts: an EPR and XPS study. J Phys Chem B 102(52), 10672–10679 (1998)
H. Chen, J. Dou, H. Xu, Removal of Cr(VI) ions by sewage sludge compost biomass from aqueous solutions: Reduction to Cr(III) and biosorption. Appl Surf Sci 425, 728–735 (2017)
A.N.Z. Alshehri, Microbial desalination cell technology for simultaneous water desalination and reduction of chromium Ion Cr. ARPN J Agri Biol Sci 12(10), 315–325 (2017)
M.P. Watts et al., Biogenic nano-magnetite and nano-zero valent iron treatment of alkaline Cr (VI) leachate and chromite ore processing residue. Appl Geochem 54, 27–42 (2015)
Electronic properties of perovskite strontium chromium oxyfluoride epitaxial thin films fabricated via low-temperature topotactic reaction. Phys Rev Mat 4(2), 025004 (2020)
E. Desimoni et al., An x-ray photoelectron spectroscopic study of some chromium–oxygen systems. Surf Interface Anal 13(2–3), 173–179 (1988)
F.R. Espinoza-Quiñones et al., Application of high resolution X-ray emission spectroscopy on the study of Cr ion adsorption by activated carbon. Appl Radiat Isot 68(12), 2208–2213 (2010)
Q. Wang et al., Impact of Fe (III) as an effective electron-shuttle mediator for enhanced Cr (VI) reduction in microbial fuel cells: reduction of diffusional resistances and cathode overpotentials. J Hazard Mater 321, 896–906 (2017)
S.H. Paiman et al., Functionalization effect of Fe-type MOF for methylene blue adsorption. J Saudi Chem Soc 24(11), 896–905 (2020)
Q.-Y. Wu et al., Hierarchically porous carbon membranes derived from PAN and their selective adsorption of organic dyes. Chin J Polym Sci 34(1), 23–33 (2016)
Bansal, R.C. and M. Goyal, Activated carbon adsorption. 2005: CRC press.
L. Wang, J. Zhanga, A. Wang, Removal of methylene blue from aqueous solution using chitosan-g-poly (acrylic acid)/montmorillonite superadsorbent nanocomposite. Colloids Surf A: Physicochem Eng Aspects 322, 47–53 (2008)
L.J. Yu, S.S. Shukla, K.L. Dorris, A. Shukla, J.L. Margrave, Adsorption of chromium from aqueous solutions by maple sawdust. J. Hazard Mater B100, 3–63 (2003)
A.J.K. Algidsawi, A study of ability of adsorption of some dyes on activated carbon from date’ S stones. Aust J Basic Appl Sci 5(11), 1397–1403 (2011)
Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J Hazard Mater B92, 263–274 (2002)
Mohanty, K., et al., Removal of chromium (VI) from dilute aqueous solutions by activated carbon developed from Terminalia arjuna nuts activated with zinc chloride. 2005. 60(11): p. 3049–3059.
Finocchio, E., et al., Chromium (VI) removal by methylated biomass of Spirulina platensis: the effect of methylation process. 2010. 156(2): p. 264–269.
Salgado-Gómez, N., M. Macedo-Miranda, and M.J.A.c.s. Olguín, Chromium VI adsorption from sodium chromate and potassium dichromate aqueous systems by hexadecyltrimethylammonium-modified zeolite-rich tuff. 2014. 95: p. 197–204.
Hamadi, N.K., et al., Adsorption kinetics for the removal of chromium (VI) from aqueous solution by adsorbents derived from used tyres and sawdust. 2001. 84(2): p. 95–105.
Hashem, T., et al., Grafting zirconium-based metal–organic framework UiO-66-NH2 nanoparticles on cellulose fibers for the removal of Cr (VI) ions and methyl orange from water. 2019. 2(9): p. 5804–5808.
Vatanpour, V., et al., Novel chitosan/polyvinyl alcohol thin membrane adsorbents modified with detonation nanodiamonds: Preparation, characterization, and adsorption performance. 2020. 13(1): p. 1731–1740.
Caravelli, A.H., L. Giannuzzi, and N.E.J.J.o.H.M. Zaritzky, Reduction of hexavalent chromium by Sphaerotilus natans a filamentous micro-organism present in activated sludges. 2008. 156(1–3): p. 214–222.
Verma, B. and C.J.K.J.o.C.E. Balomajumder, Fabrication of magnetic cobalt ferrite nanocomposites: an advanced method of removal of toxic dichromate ions from electroplating wastewater. 2020. 37: p. 1157–1165.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All authors have agreed with the content, and all have given explicit consent to publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abuzalat, O., Wong, D. & Elsayed, M.A. Nano-Porous Composites of Activated Carbon–Metal Organic Frameworks (Fe-BDC@AC) for Rapid Removal of Cr (VI): Synthesis, Adsorption, Mechanism, and Kinetics Studies. J Inorg Organomet Polym 32, 1924–1934 (2022). https://doi.org/10.1007/s10904-022-02237-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-022-02237-9