Abstract
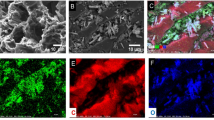
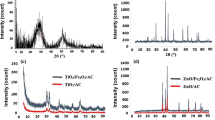
Specific industrial activities such as hydraulic fracturing generate wastewaters containing boron which exceed the standard concentration limits. Removal or reducing the concentration of this pollutant through proper treatment prior to discharge into the environment has become a critical issue. As a novel composite adsorbent, Mn-doped Fe2O4 nanoparticles loaded on activated carbon was synthesized for the first time and used for the adsorption of boron ions from aqueous solutions. This novel adsorbent was characterized by FTIR, SEM, and XRD analyses. The effect of solution pH, adsorbent dosage, temperature, initial concentration of boron ions, and contact time was studied through adsorption batch experiments and an acceptable maximum removal efficiency which was equal to 96.44% was achieved in the following conditions: pH, 6.0; adsorbent dosage, 0.1 g/L; 298.15 K; initial concentration of 1 mg/L and 85 min. Furthermore, regeneration of the adsorbent approved its performance for 3 adsorption/desorption cycles. Additionally, it was shown that the adsorption of boron ions was well described by the Temkin isotherm model and the maximum adsorption capacity of 9.03 mg/g was also achieved. The values of RL and n were 0.05 and 0.29 which denote that the adsorption was desirable, reversible, and chemical. Kinetic study showed that pseudo-second-order model (R2 = 0.9997) explained the kinetic behavior of the process well and based on the thermodynamic analysis the current adsorption process was exothermic and spontaneous (ΔH° = −6.2 kJ/mol and ΔS° kJ/mol·K= −16.11). These results indicated that pretreatment of Mn-doped Fe2O4 nanoparticles loaded on activated carbon can optimize the removal of boron ions from aqueous solution since this novel adsorbent presented an acceptable adsorptive performance in this study and it can be recommended for the removal of similar pollutants from wastewaters.










Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mishra S, Bharagava RN, More N et al (2019) Heavy metal contamination: an alarming threat to environment and human health. In: Environmental biotechnology: For sustainable future. Springer, pp 103–125
Hembrom S, Singh B, Gupta SK, Nema AK (2020) A comprehensive evaluation of heavy metal contamination in foodstuff and associated human health risk: a global perspective. In: Contemporary environmental issues and challenges in era of climate change. Springer, pp 33–63
Rai PK (2009) Heavy metal phytoremediation from aquatic ecosystems with special reference to macrophytes. Crit Rev Environ Sci Technol 39:697–753
Chary NS, Kamala CT, Raj DSS (2008) Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol Environ Saf 69:513–524
Khedri A, Jafari D, Esfandyari M (2021) Adsorption of nickel (II) ions from synthetic wastewater using activated carbon prepared from Mespilus germanica leaf. Arab J Sci Eng 47:6155–6166
Ismail MHS, Zhang X, Lazim MFM (2013) Removal of boron and arsenic from petrochemical wastewater by using aquatic booster as adsorbent. Pol J Environ Stud 22:403–408
Sari MA, Chellam S (2015) Mechanisms of boron removal from hydraulic fracturing wastewater by aluminum electrocoagulation. J Colloid Interface Sci 458:103–111
Thompson R (1974) Industrial applications of boron compounds. Pure Appl Chem 39:547–559
Organization WH (2009) Boron in drinking-water: background document for development of WHO Guidelines for Drinking-water Quality. World Health Organization. https://apps.who.int/iris/handle/10665/70170
Shih Y-J, Liu C-H, Lan W-C, Huang Y-H (2014) A novel chemical oxo-precipitation (COP) process for efficient remediation of boron wastewater at room temperature. Chemosphere 111:232–237
Rahmawati K, Ghaffour N, Aubry C, Amy GL (2012) Boron removal efficiency from Red Sea water using different SWRO/BWRO membranes. J Membr Sci 423:522–529
Yılmaz AE, Boncukcuoglu R, Yılmaz MT, Kocakerim MM (2005) Adsorption of boron from boron-containing wastewaters by ion exchange in a continuous reactor. J Hazard Mater 117:221–226
Yürüm A, Taralp A, Bıçak N et al (2013) High performance ligands for the removal of aqueous boron species by continuous polymer enhanced ultrafiltration. Desalination 320:33–39
Mirbagheri SA, Hosseini SN (2005) Pilot plant investigation on petrochemical wastewater treatmentfor the removal of copper and chromium with the objective of reuse. Desalination 171:85–93
Kluczka J, Trojanowska J, Zolotajkin M et al (2007) Boron removal from wastewater using adsorbents. Environ Technol 28:105–113
Öztürk N, Kavak D (2005) Adsorption of boron from aqueous solutions using fly ash: batch and column studies. J Hazard Mater 127:81–88
Karahan S, Yurdakoç M, Seki Y, Yurdakoç K (2006) Removal of boron from aqueous solution by clays and modified clays. J Colloid Interface Sci 293:36–42
Al. Haddabi M, Ahmed M, Al. Jebri Z et al (2016) Boron removal from seawater using date palm (Phoenix dactylifera) seed ash. Desalin Water Treat 57:5130–5137
Papageorgiou SK, Katsaros FK, Kouvelos EP et al (2006) Heavy metal sorption by calcium alginate beads from Laminaria digitata. J Hazard Mater 137:1765–1772
Jalali M, Rajabi F, Ranjbar F (2016) The removal of boron from aqueous solutions using natural and chemically modified sorbents. Desalin Water Treat 57:8278–8288
Kluczka J, Pudło W, Krukiewicz K (2019) Boron adsorption removal by commercial and modified activated carbons. Chem Eng Res Des 147:30–42
Melliti A, Kheriji J, Bessaies H, Hamrouni B (2020) Boron removal from water by adsorption onto activated carbon prepared from palm bark: kinetic, isotherms, optimisation and breakthrough curves modeling. Water Sci Technol 81:321–332
Polowczyk I, Ulatowska J, Koźlecki T et al (2013) Studies on removal of boron from aqueous solution by fly ash agglomerates. Desalination 310:93–101
Mariana M, Abdul Khalil HPS, Mistar EM et al (2021) Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption. J Water Process Eng 43:102221
Ojha A, Tiwary D, Oraon R, Singh P (2021) Degradations of endocrine-disrupting chemicals and pharmaceutical compounds in wastewater with carbon-based nanomaterials: a critical review. Environ Sci Pollut Res 28:30573–30594
Soffian MS, Halim FZA, Aziz F et al (2022) Carbon-based material derived from biomass waste for wastewater treatment. Environ Adv 9:100259
Cullity BD (1956) Elements of X-ray diffraction. Addison-Wesley Publishing
Sarojini G, Venkateshbabu S, Rajasimman M (2021) Facile synthesis and characterization of polypyrrole-iron oxide–seaweed (PPy-Fe3O4-SW) nanocomposite and its exploration for adsorptive removal of Pb (II) from heavy metal bearing water. Chemosphere 278:130400
Rahmani Piani M, Abrishamkar M, Mombeni Goodajdar B (2021) Removal Phenol from aqueous solutions using environmental friendly and effective adsorbent onto Mn-doped Fe2O4 nanoparticles loaded on activated carbon. J Phys Theor Chem 17:75–92
Ma M, Lu Y, Chen R et al (2014) Hexavalent chromium removal from water using heat-acid activated red mud. Open J Appl Sci 4(5):275–284
Aboli E, Jafari D, Esmaeili H (2020) Heavy metal ions (lead, cobalt, and nickel) biosorption from aqueous solution onto activated carbon prepared from Citrus limetta leaves. CARBON Letters 30(6)
Ozdes D, Gundogdu A, Kemer B et al (2009) Removal of Pb (II) ions from aqueous solution by a waste mud from copper mine industry: equilibrium, kinetic and thermodynamic study. J Hazard Mater 166:1480–1487
Al-Ghouti MA, Khan M, Malik A et al (2022) Development of novel nano-γ-Al2O3 adsorbent from waste aluminum foil for the removal of boron and bromide from aqueous solution. J Water Process Eng 50:103312
García FE, Plaza-Cazón J, Montesinos VN et al (2018) Combined strategy for removal of Reactive Black 5 by biomass sorption on Macrocystis pyrifera and zerovalent iron nanoparticles. J Environ Manag 207:70–79
Takdastan A, Mahvi AH, Lima EC et al (2016) Preparation, characterization, and application of activated carbon from low-cost material for the adsorption of tetracycline antibiotic from aqueous solutions. Water Sci Technol 74:2349–2363
Davila-Jimenez MM, Elizalde-González MP, Geyer W et al (2003) Adsorption of metal cations from aqueous solution onto a natural and a model biocomposite. Colloids Surfaces A Physicochem Eng Asp 219:243–252
Dehghani MH, Dehghan A, Alidadi H et al (2017) Removal of methylene blue dye from aqueous solutions by a new chitosan/zeolite composite from shrimp waste: kinetic and equilibrium study. Korean J Chem Eng 34:1699–1707
Kumar IA, Viswanathan N (2018) Preparation and testing of a tetra-amine copper (II) chitosan bead system for enhanced phosphate remediation. Carbohydr Polym 183:173–182
Song Q, Wang H, Yang B et al (2016) A novel adsorbent of Ag-FMWCNTs for the removal of SMX from aqueous solution. RSC Adv 6:75855–75861
Gimbert F, Morin-Crini N, Renault F et al (2008) Adsorption isotherm models for dye removal by cationized starch-based material in a single component system: error analysis. J Hazard Mater 157:34–46
Johnson RD, Arnold FH (1995) The Temkin isotherm describes heterogeneous protein adsorption. Biochim Biophys Acta 1247:293–297
Madbouly M, Al-Anwar A (2018) Tamarix aphylla biomass as efficient adsorbent for removal of Pb (II) ions from aqueous solution: kinetic and applicability for different isotherm models. Egypt J Chem 61:101–119
Yan J, Li R (2022) Simple and low-cost production of magnetite/graphene nanocomposites for heavy metal ions adsorption. Sci Total Environ 813:152604
Bazargan-Lari R, Zafarani HR, Bahrololoom ME, Nemati A (2014) Removal of Cu (II) ions from aqueous solutions by low-cost natural hydroxyapatite/chitosan composite: equilibrium, kinetic and thermodynamic studies. J Taiwan Inst Chem Eng 45:1642–1648
Kluczka J, Korolewicz T, Zołotajkin M et al (2013) A new adsorbent for boron removal from aqueous solutions. Environ Technol 34:1369–1376
Zelmanov G, Semiat R (2014) Boron removal from water and its recovery using iron (Fe+ 3) oxide/hydroxide-based nanoparticles (NanoFe) and NanoFe-impregnated granular activated carbon as adsorbent. Desalination 333:107–117
Kluczka J, Trojanowska J, Zołotajkin M (2015) Utilization of fly ash zeolite for boron removal from aqueous solution. Desalin Water Treat 54:1839–1849
Yüksel S, Yürüm Y (2009) Removal of boron from aqueous solutions by adsorption using fly ash, zeolite, and demineralized lignite. Sep Sci Technol 45:105–115
Author information
Authors and Affiliations
Contributions
Gholamhossien Vatankhah: draft manuscript preparation, experimental, analysis, and interpretation of results
Farshid Parsa: draft manuscript preparation, methodology, project administration, software, editing
Dariush Jafari: simulation, experimental, methodology, writing—review and editing
Morteza Esfandyari: methodology, project administration, software, validation, writing—original draft
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vatankhah, G., Parsa, F., Jafari, D. et al. Evaluation of adsorptive performance of Mn-doped Fe2O4 nanoparticles loaded on activated carbon in removal of boron ions from synthetic wastewater. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04695-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04695-8