Abstract
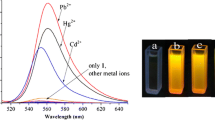
A coumarin-appended rhodamine derivative was prepared by reacting rhodamine hydrazide and coumarin-3-carboxylic acid, which fluorescence sensing behavior toward Zn2+ against other metal ions was investigated in CH3CN. Significantly, the rodamine-coumarin derivative exhibited highly selective and sensitive recognition toward Zn2+ with a limit of detection (LOD) down to 10−9 M. Upon addition of Zn2+, remarkable fluorescent intensities enhanced and also clear color changed from colorless to pink. The Job’s plot indicated the formation of 1:1 complex between the rhodamine-coumarin derivative and Zn2+. The presence of common coexisting alkali, alkaline earth, and transition metal ions showed small or no interference with the detection of Zn2+. The conjugate dye could be used for “naked-eye” detection of Zn2+.








Similar content being viewed by others
References
Parkesh R, Lee TC, Gunnlaugsson T (2007) Highly selective 4-amino-1,8-naphthalimide based fluorescent photoinduced electron transfer (PET) chemosensors for Zn(II) under physiological pH conditions. Org Biomol Chem 5:310–317
Hirano T, Kikuchi K, Urano Y, Higuchi T, Nagano T (2000) Highly zinc-selective fluorescent sensor molecules suitable for biological applications. J Am Chem Soc 122:12399–12400
Xu Z, Baek KH, Kim HN, Cui J, Qian X, Spring DR, Shin I, Yoon J (2009) Zn2+-triggered amide tautomerization produces a highly Zn2+-selective, cell-permeable, and ratiometric fluorescent sensor. J Am Chem Soc 132:601–610
Meng XM, Zhu MZ, Liu L, Guo QX (2006) Novel highly selective fluorescent chemosensors for Zn(II). Tetrahedron Lett 47:1559–1562
Ajayaghosh A, Carol P, Sreejith SN (2005) A ratiometric, fluorescence probe for selective visual sensing of Zn2+. J Am Chem Soc 127:14962–14963
Frederickson CJ, Koh JY, Bush AI (2005) The neurobiology of zinc in health and disease. Nat Rev Neurosci 6:449–462
Li M, Lu HY, Liu RL, Chen JD, Chen CF (2012) Turn-on fluorescent sensor for selective detection of Zn2+, Cd2+, and Hg2+ in water. J Org Chem 77:3670–3673
Qian F, Zhang C, Zhang Y, He W, Gao X, Hu P, Guo Z (2009) Visible light excitable Zn2+ fluorescent sensor derived from an intramolecular charge transfer fluorophore and its in vitro and in vivo application. J Am Chem Soc 131:1460–1468
Dakanali M, Roussakis E, Kay AR, Katerinopoulos HE (2005) Synthesis and photophysical properties of a fluorescent TREN type ligand incorporating the coumarin chromophore and its zinc complex. Tetrahedron Lett 46:4193–4196
Yu H, Li G, Zhang B, Zhang X, Xiao Y, Wang J, Song Y (2016) A neutral pH probe of rhodamine derivatives inspired by effect of hydrogen bond on pKa and its organelle-targetable fluorescent imaging. Dyes Pigments 133:93–99
Kikuchi K, Komatsu H, Nagano T (2004) Zinc sensing for cellular application. Curr Opin Chem Biol 8:182–191
Carol P, Sreejith S, Ajayaghosh A (2007) Ratiometric and near-infrared molecular probes for the detection and imaging of zinc ions. Chem Asian J 2:338–348
Nolan EM, Lippard SJ (2009) Small-molecule fluorescent sensors for investigating zinc metalloneurochemistry. Acc Chem Res 42:193–203
Yuasa J, Mitsui A, Kawai T (2011) π–π* emission from a tetrazine derivative complexed with zinc ion in aqueous solution: a unique water-soluble fluorophore. Chem Commun 47:5807–5809
Zhang Y, Guo X, Si W, Jia L, Qian X (2008) Ratiometric and water-soluble fluorescent zinc sensor of carboxamidoquinoline with an alkoxyethylamino chain as receptor. Org Lett 10:473–476
Xue L, Liu C, Jiang H (2009) Highly sensitive and selective fluorescent sensor for distinguishing cadmium from zinc ions in aqueous media. Org Lett 11:1655–1658
Kim HN, Lee MH, Kim HJ, Kim JS, Yoon J (2008) A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions. Chem Soc Rev 37:1465–1472
Shi W, Ma H (2008) Rhodamine B thiolactone: a simple chemosensor for Hg2+ in aqueous media. Chem Commun:1856–1858
Dujols V, Ford F, Czarnik AW (1997) A long-wavelength fluorescent chemodo-simeter selective for Cu(II) ion in water. J Am Chem Soc 119:7386–7387
Xiang Y, Tong A (2006) A new rhodamine-based chemosensor exhibiting selective Fe(III)-amplified fluorescence. Org Lett 8:1549–1552
Kwon JY, Jang YJ, Lee YJ, Kim K, Seo M, Nam W, Yoon J (2005) A highly selective fluorescent chemosensor for Pb2+. J Am Chem Soc 127:10107–10111
Huang K, Yang H, Zhou Z, Yu M, Li F, Gao Z, Yi T, Huang C (2008) Multisignal chemosensor for Cr3+ and its application in bioimaging. Org Lett 10:2557–2560
Tang Y, Cui S, Pu S (2016) A dual-channel sensor for Hg2+ based on a diarylethene with a rhodamine B unit. J Fluoresc 26:1421–1429
Tang L, Li F, Liu M, Nandhakumar R (2011) A new rhodamine B-coumarin fluorochrome for colorimetric recognition of Cu2+ and fluorescent recognition of Fe3+ in aqueous media. Bull Kor Chem Soc 32:3400–3404
Wu JS, Liu WM, Zhuang XQ, Wang F, Wang PF, Tao SL, Zhang XH, Wu SK, Lee ST (2007) Fluorescence turn on of coumarin derivatives by metal cations: a new signaling mechanism based on C = N isomerization. Org Lett 9:33–36
Xiang Y, Tong AJ, Jin PY, Ju Y (2006) New fluorescent rhodamine hydrazone chemosensor for Cu(II) with high selectivity and sensitivity. Org Lett 8:2863–2866
Jiang BP, Tan X, Shen XC, Lei WQ, Liang WQ, Ji SC, Liang H (2016) One-step fabrication of a multifunctional aggregation-induced emission nanoaggregate for targeted cell imaging and enzyme-triggered cancer chemotherapy. ACS Macro Lett 5:450–454
Zhang YH, Zhang YM, Chen Y, Yang Y, Liu Y (2014) Phenanthroline bridged bis(β-cyclodextrin)s/ adamantane-carboxylic acid supramolecular complex as an efficient fluorescence sensor to Zn2+. Org Chem Front 1:355–360
Wang KP, Chen Y, Liu Y (2015) A polycation-induced secondary assembly of amphiphilc calixarene and its multi-stimuli responsive gelation behavior. Chem Commun 51:1647–1649
Yuan MJ, Zhou WD, Liu XF, Zhu M, Li JB, Yin XD, Zheng HY, Zuo ZC, Ouyang CB, Liu HB, Li YL, Zhu DB (2008) A multianalyte chemosensor on a single molecule: promising structure for an integrated logic gate. J Org Chem 73:5008–5014
Dhara K, Karan S, Ratha J, Roy P, Chandra G, Manassero M, Mallik B, Banerjee P (2007) A two-dimensional coordination compound as a zinc ion selective luminescent probe for biological applications. Chem Asian J 2:1091–1100
Hu ZQ, Wang XM, Feng YC, Ding L, Lu HY (2011) Sulfonyl rhodamine hydrazide: a sensitive and selective chromogenic and flurescent chemodosimeter for copper ion in aqueous media. Dyes Pigments 88:257–261
Hu ZQ, Li M, Liu MD, Zhuang WM, Li GK (2013) A highly sensitive fluorescent acidic pH probe based on rhodamine B diethyl-2-aminobutenedioate conjugate and its application in living cells. Dyes Pigments 96:71–75
Li G, Tang J, Ding P, Ye Y (2016) A rhodamine-benzimidazole based chemosensor for Fe3+ and its application in living cells. J Fluoresc 26:155–161
Acknowledgements
We are grateful to the National Natural Science Foundation of China (21172127) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, KP., Jin, ZH., Shang, HS. et al. A highly Selective Fluorescent Chemosensor for Zn2+ Based on the Rhodamine Derivative Incorporating Coumarin Group. J Fluoresc 27, 629–633 (2017). https://doi.org/10.1007/s10895-016-1991-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1991-0