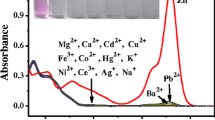
Abstract
A new rhodamine-based fluorescent chemosensor (1) has been designed and synthesized by linking rhodamine 6G hydrazide with N-methylisatin via an imine linkage. The receptor can selectively recognize and sense Pb2+, Hg2+ and Cd2+ by showing different fluorescence characteristics. In ethanol/HEPES buffer medium, the addition of Cd2+ caused a yellowish-green fluorescence, while the presence of Pb2+ or Hg2+ gave rise to an orange fluorescence. Additionally, the sensor shows an irreversible fluorescence response to Pb2+ and reversible fluorescence responses to Hg2+ and Cd2+.






Similar content being viewed by others
References
Finkelstein Y, Markowitz ME, Rosen JF (1998) Low-level lead-induced neurotoxicity in children: an update on central nervous system effects. Brain res rev 27:168–176. doi:10.1016/S0165-0173(98)00011-3
Bolger PM, Schwetz BA (2002) Mercury and health. N Engl J med 347:1735–1736. doi:10.1056/NEJMp020139
Waisberg M, Joseph P, Hale B, Beyersmann D (2003) Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 192:95–117. doi:10.1016/S0300-483X(03)00305-6
Carter KP, Young AM, Palmer AE (2014) Fluorescent sensors for measuring metal ions in living systems. Chem rev 114:4564–4601. doi:10.1021/cr400546e
Jeong Y, Yoon J (2012) Recent progress on fluorescent chemosensors for metal ions. Inorg Chim Acta 381:2–14. doi:10.1016/j.ica.2011.09.011
Kim HN, Ren WX, Kim JS, Yoon J (2012) Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem Soc rev 41:3210–3244. doi:10.1039/C1CS15245A
Xu L, Xu Y, Zhu W, Sun X, Xu Z, Qian X (2012) Modulating the selectivity by switching sensing media: a bifunctional probe selectivity for Cd2+ and Pb2+ in different aqueous solutions. RSC Adv 2:6323–6328. doi:10.1039/C2RA20840G
Lv Y, Wu L, Shen W, Wang J, Xuan G, Sun X (2015) A porphyrin-based chemosensor for colorimetric and fluorometric detection of cadmium(II) with high selectivity. J Porphyr Phthalocya 19:769–774. doi:10.1142/S1088424615500510
Liu Q, Feng L, Yuan C, Zhang L, Shuang S, Dong C, Hu Q, Choi MMF (2014) A highly selective fluorescent probe for cadmium ions in aqueous solution and living cells. Chem Commun 50:2498–2501. doi:10.1039/C3CC48668K
Cheng T, Wang T, Zhu W, Chen X, Yang Y, Xu Y, Qian X (2011) A simple highly sensitive and selective TURN-ON fluorescent chemosensor for the detection of cadmium ions in physiological conditions. OrgLett 13:3656–3659. doi:10.1021/ol201305d
Kim HN, Lee MH, Kim HJ, Kim JS, Yoon J (2008) A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions. Chem Soc rev 37:1465–1472. doi:10.1039/B802497A
Chen X, Pradhan T, Wang F, Kim JS, Yoon J (2012) Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives. Chem rev 112:1910–1956. doi:10.1021/cr200201z
Wang M, Yan F, Zou Y, Chen L, Yang N, Zhou X (2014) Recognition of Cu2+ and Hg2+ in physiological conditions by a new rhodamine based dual channel fluorescent probe. Sensors Actuators B Chem 192:512–521. doi:10.1016/j.snb.2013.11.031
Hua J, Hua Z, Cui Y, Zhang X, Gao H-W, Uvdal K (2014) A rhodamine-based fluorescent probe for Hg2+ and its application for biological visualization. Sensors Actuators B Chem 203:452–458. doi:10.1016/j.snb.2014.06.104
Chen X, Meng X, Wang S, Cai Y, Wu Y, Feng Y, Zhua M, Guo Q (2013) A rhodamine-based fluorescent probe for detecting Hg2+ in a fully aqueous environment. Dalton Trans 42:14819–14825. doi:10.1039/C3DT51279G
Kwon JY, Jang YJ, Lee YJ, Kim KM, Seo MS, Nam W, Yoon J (2005) A highly selective fluorescent chemosensor for Pb2+. J am Chem Soc 127:10107–10111. doi:10.1021/ja051075b
Ghosh K, Sarkar T, Majumdar A, Mandal SK, Khuda-Bukhsh AR (2014) Rhodamine-labelled simple architectures for fluorometric and colorimetric sensing of Hg2+ and Pb2+ ions in semi-aqueous and aqueous environments. Anal Methods 6:2648–2654. doi:10.1039/C4AY00217B
Goswami S, Aich K, Das S, Das AK, Manna A, Halder S (2013) A highly selective and sensitive probe for colorimetric and fluorogenic detection of Cd2+ in aqueous media. Analyst 138:1903–1907. doi:10.1039/C3AN36884J
Aich K, Goswami S, Das S, Mukhopadhyay CD, Quah CK, Fun H-K (2015) Cd2+ triggered the FRET “ON”: a new molecular switch for the ratiometric detection of Cd2+ with live-cell imaging and bound X-ray structure. Inorg Chem 54:7309–7315. doi:10.1021/acs.inorgchem.5b00784
Sheldrick GM (1997) SHELXS-97, Program for crystal structure solution. University of Göttingen, Germany
Sheldrick GM (1997) SHELXL-97, Program for crystal structure refinement. University of Göttingen, Germany
Zhang Z, Zheng Y, Hang W, Yan X, Zhao Y (2011) Sensitive and selective off-on rhodamine hydrazide fluorescent chemosensor for hypochlorous acid detection and bioimaging. Talanta 85:779–786. doi:10.1016/j.talanta.2011.04.078
Goswami S, Paul S, Manna A (2013) Selective “naked eye” detection of al(III) and PPi in aqueous media on a rhodamine-isatin hybrid moiety. RSC Adv 3:10639–10643
Yang Y-K, Yook K-J, Tae J (2005) A rhodamine-based fluorescent and colorimetric chemodosimeter for the rapid detection of Hg2+ ions in aqueous media. J am Chem Soc 127:16760–16761. doi:10.1039/C3RA40984H
Acknowledgments
This work is supported by the National Natural Science Foundation of China (21162010), the Natural Science Foundation of Hainan Province (No. 20162028) and Program for Innovative Research Team in University (IRT-16R19).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 884 kb)
Rights and permissions
About this article
Cite this article
Su, W., Yuan, S. & Wang, E. A Rhodamine-Based Fluorescent Chemosensor for the Detection of Pb2+, Hg2+ and Cd2+ . J Fluoresc 27, 1871–1875 (2017). https://doi.org/10.1007/s10895-017-2124-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2124-0