Abstract
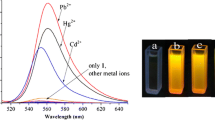
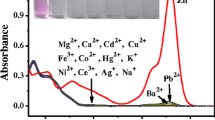
The photophysical and complexing properties of Rhod-5N (commercially available) in MOPS buffer are reported. This fluorescent molecular sensor consists of a BAPTA chelating moiety bound to a rhodamine fluorophore. Its fluorescence quantum yield is low and a drastic enhancement of fluorescence intensity upon cation binding was observed. Special attention was paid to the complexation with Cd2+, a well known toxic metal ion. Possible interference with other metal ions (Na+, K+, Mg2+, Ca2+, Zn2+, Pb2+) was examined. Rhod-5N was found to be highly selective of Cd2+ over those interfering cations except Pb2+. The limit of detection is 3.1 μg l−1.






Similar content being viewed by others
References
Dobson S (1992) Cadmium-environmental aspects. World Health Organization, Geneva
World Health Organization (2004) Guidelines for drinking water quality, 3rd edition. 1, 491
Valeur B (1994) Principles of fluorescent probe design for ion recognition. In: Lakowicz JR (ed) Probe design and chemical sensing, topics in fluorescence spectroscopy, vol. 4. Plenum, New York, pp 21–48
Desvergne J-P, Czarnik AW (Eds) (1997) Chemosensors of Ion and Molecular Recognition, NATO ASI Series. Kluwer Academic, Dordrecht, vol. C492
de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997) Signaling recognition events with fluorescent sensors and switches. Coord Chem Rev 97:1515–1566
Valeur B, Leray I (2000) Design principles of fluorescent molecular sensors for cation recognition. Coord Chem Rev 205:3–40
Valeur B (2002) Molecular fluorescence. Principles and applications. Wiley-VCH, Weinheim (chap. 10)
Fluorescent sensors, special issue of the Journal of Materials Science, vol. 15 (2005), Guest editors: A. P. de Silva and P. Tecilla
Hefley AJ, Jaselskis B (1974) Fluorometric determination of submicrogram quantities of cadmium by reaction with the metallofluorochromic reagent, calcein. Anal Chem 46:2036–2038
Charles S, Dubois F, Yunus S, Vander Donckt E (2000) Determination by fluorescence spectroscopy of cadmium at the subnanomolar level: application to seawater. J Fluorescence 10:99–105
Luo HY, Jiang JH, Zhang XB, Li CY, Shen GL, Yu RQ (2007) Synthesis of porphyrin-appended terpyridine as a chemosensor for cadmium based on fluorescent enhancement. Talanta 72:575–581
Hennrich G, Sonnenschein H, Resch-Genger U (1999) Redox switchable fluorescent probe selective for either Hg(II) or Cd(II) and Zn(II). J Am Chem Soc 121:5073–5074
Gunnlaugsson T, Lee TC, Parkesh R (1999) Cd(II) sensing in water using novel aromatic iminodiacetate based fluorescent chemosensors. Org Lett 121:2158–4068
Peng X, Du J, Fan J, Wang J, Wu Y, Zhao J, Sun S, Xu T (2007) A selective fluorescent sensor for imaging Cd2+ in living cells. J Am Chem Soc 1298:1500–1501
Lu C, Xu Z, Cui J (2007) Ratiometric and highly selective fluorescent sensor for cadmium under physiological pH range: a new strategy to discriminate cadmium from zinc. J Org Chem 72:3554–3557
Bronson RT, Michaelis DJ, Lamb RD, Husseini GA, Farnsworth PB, Linford MR, Izatt RM, Bradshaw JS, Savage PB (2005) Efficient immobilization of a cadmium chemosensor in a thin film: generation of a cadmium sensor prototype. Org Lett 7:1105–1108
Gunnlaugsson T, Lee TC, Parkesh R (2003) Cd(II) sensing in water using novel aromatic iminodiacetate based fluorescent chemosensors. Org Lett 5:4065–4068
Costero AM, Andreu R, Monrab E, Martinez-Manez R, Sancenon F, Soto J (2002) 4,4¢-Bis(dimethylamino)biphenyl containing binding sites. A new fluorescent subunit for cation sensing.. J Chem Soc Dalton Trans 8:1769–1775
Choi M, Kim M, Lee KD, Han KN, Yoon IA, Chung HJ, Yoon J (2001) A new reverse PET chemosensor and its chelatoselective aromatic cadmiation. Org Lett 3:3455–3457
Prodi L, Bolletta F, Montalti M, Zaccheroni N (1999) Searching for new luminescent sensors. Synthesis and photophysical properties of a tripodal ligand incorporating the dansyl chromophore and of its metal complexes. Eur J Inorg Chem 3:455–460
Huston ME, Engleman C, Czarnik AW (1990) Chelatoselective fluorescence perturbation in anthrylazamacrocycle conjugate probes. Electrophilic aromatic cadmiation. J Am Chem Soc 112:7054–7056
Akkaya EU, Huston ME, Czarnik AW (1990) Chelation-enhanced fluorescence of anthrylazamacrocycle conjugate probes in aqueous solution. J Am Chem Soc 112:3590–3593
Haugland RP (2005) The handbook: a guide to fluorescent probes and labelling techniques, chap. 19, 10th edn. Invitrogen, USA
Boens N, Qin W, Basaric N, Hofkens J, Ameloot M, Pouget J, Lefevre JP, Valeur B, Gratton E, vandeVen M, Silva Jr ND, Engelborghs Y, Willaert K, Sillen A, Rumbles G, Phillips D, Visser AJWG, van Hoek A, Lakowicz JR, Malak H, Gryczynski I, Szabo AG, Krajcarski DT, Tamai N, Miura A (2007) Fluorescence lifetime standards for time and frequency domain fluorescence spectroscopy. Anal Chem 79:2137–2149
Gampp H, Maeder M, Meyer CJ, Zuberbühler AD (1985) Calculation of equilibrium constants from multiwavelength spectroscopic data—I: Mathematical considerations. Talanta 32:95–101
Plaza P, Dai Hung N, Martin MM, Meyer YH, Vogel M, Rettig W (1992) Ultrafast internal charge transfer in a donor-modified rhodamine. Chem Phys 168:365–373
McNamara CJ, Perry TD IV, Bearce K, Hernandez-Duque G, Mitchell R (2005) Measurement of limestone biodeterioration using the Ca2+ binding fluorochrome Rhod-5N. J Microbiol Methods 61:245–250
Zhao X, Yoshida M, Brotto L, Takeshima H, Weisleder N, Hirata Y, Nosek TM, Ma J, Brotto M (2005) Enhanced resistance to fatigue and altered calcium handling properties of sarcalumenin knockout mice. Physiol Genomics 23:72–78
Ribou AC, Salmon JM, Vigo J, Goyet C (2007) Measurements of calcium with a fluorescent probe Rhod-5N: Influence of high ionic strength and pH. Talanta 71:437–442
Phung-Nguyen AT, Hernando V, Rieutord A, Brion F, Prognon P (2006) Are fluorescent calcium probes useful for studying amino acid-cation interactions in total nutritional admixtures? Luminescence 21:356–357
Rand MD, Lindblom A, Carlson J, Villoutreix BO, Stenflo J (1997) Calcium binding to tandem repeats of EGF-like modules. Expression and characterization of the EGF-like module of human Notch-1 implicated in receptor-ligand interactions.. Protein Science 6:2059–2071
Schoutteten L, Denjean P, Faure J, Pansu RB (1999) Photophysics of Calcium Green 1 in vitro and in live cells. Phys Chem Chem Phys 1:2463–2469
Tessier A, Méallet-Renault R, Denjean P, Miller D, Pansu RB (1999) Conformational dynamics of Calcium Green 1 by fluorescence correlation spectroscopy. Phys Chem Chem Phys 1:5767–5769
Gaillard S, Yakolev A, Luccardini C, Oheim M, Feltz A, Mallet JM (2007) Synthesis and characterization of a red-emitting Ca2+ indicator, calcium ruby. Org Lett 9:2629–2632
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soibinet, M., Souchon, V., Leray, I. et al. Rhod-5N as a Fluorescent Molecular Sensor of Cadmium(II) Ion. J Fluoresc 18, 1077–1082 (2008). https://doi.org/10.1007/s10895-008-0352-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-008-0352-z