Abstract
Plants damaged by insect herbivory often respond by inducing a suite of defenses that can negatively affect an insect’s growth and fecundity. Ostrinia nubilalis (European corn borer, ECB) is one of the most devastating insect pests of maize, and in the current study, we examined the early biochemical changes that occur in maize stems in response to ECB herbivory and how these rapidly induced defenses influence the growth of ECB. We measured the quantities of known maize defense compounds, benzoxazinoids and the kauralexin class of diterpenoid phytoalexins. ECB herbivory resulted in decreased levels of the benzoxazinoid, 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one)-β-D-glucopyranose (DIMBOA-Glc), and a corresponding increase in 2-(2-hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one)-β-D-glucopyranose (HDMBOA-Glc). Total quantities of benzoxazinoids and kauralexins were increased as early as 24 h after the initiation of ECB feeding. The plant hormones, jasmonic acid (JA) and ethylene (ET), and the transcripts encoding their key biosynthetic enzymes also accumulated in response to ECB herbivory, consistent with a role in defense regulation. The combined pharmacological application of JA and the ET precursor, 1-aminocyclopropane-1-carboxylic acid to stem internode tissue likewise resulted in changes in benzoxazinoids similar to that observed with ECB damage. Despite the fact that maize actively mounts a defense response to ECB stem feeding, no differences in percent weight gain were observed between ECB larvae that fed upon non-wounded control tissues compared to tissues obtained from plants previously subjected to 24 h ECB stem herbivory. These rapid defense responses in maize stems do not appear to negatively impact ECB growth, thus suggesting that ECB have adapted to these induced biochemical changes.





Similar content being viewed by others
References
Abe, H., Ohnishi, J., Narusaka, M., Seo, S., Narusaka, Y., Tsuda, S., and Kobayashi, M. 2008. Function of jasmonate in response and tolerance of Arabidopsis to thrip feeding. Plant Cell Physiol. 491:68–80.
Abe, H., Shimoda, T., Ohnishi, J., Kugimiya, S., Narusaka, M., Seo, S., Narusaka, Y., Tsuda, S., and Kobayashi, M. 2009. Jasmonate-dependent plant defense restricts thrips performance and preference. BMC Plant Biol. 9.
Agrawal, A. A. 1999. Induced responses to herbivory in wild radish: effects on several herbivores and plant fitness. Ecology 805:1713–1723.
Baldwin, I. T., Zhang, Z. P., Diab, N., Ohnmeiss, T. E., McCloud, E. S., Lynds, G. Y., and Schmelz, E. A. 1997. Quantification, correlations and manipulations of wound-induced changes in jasmonic acid and nicotine in Nicotiana sylvestris. Planta 2014:397–404.
Bruinsma, M., Van Dam, N. M., Van Loon, J. J. A., and Dicke, M. 2007. Jasmonic acid-induced changes in Brassica oleracea affect oviposition preference of two specialist herbivores. J. Chem. Ecol. 334:655–668.
Brunissen, L., Vincent, C., Le Roux, V., and Giordanengo, P. 2010. Effects of systemic potato response to wounding and jasmonate on the aphid Macrosiphum euphorbiae (Sternorryncha: Aphididae). J. App. Entomol. 1347:562–571.
Cambier, V., Hance, T., and de Hoffmann, E. 2000. Variation of DIMBOA and related compounds content in relation to the age and plant organ in maize. Phytochemistry 532:223–229.
Cambier, V., Hance, T., and De Hoffmann, E. 2001. Effects of 1,4-benzoxazin-3-one derivatives from maize on survival and fecundity of Metopolophium dirhodum (Walker) on artificial diet. J. Chem. Ecol. 272:359–370.
Erb, M., Flors, V., Karlen, D., de Lange, E., Planchamp, C., D’Alessandro, M., Turlings, T. C. J., and Ton, J. 2009. Signal signature of aboveground-induced resistance upon belowground herbivory in maize. Plant J. 592:292–302.
Frey, M., Chomet, P., Glawischnig, E., Stettner, C., Grun, S., Winklmair, A., Eisenreich, W., Bacher, A., Meeley, R. B., Briggs, S. P., Simcox, K., and Gierl, A. 1997. Analysis of a chemical plant defense mechanism in grasses. Science 277:696–699.
Frey, M., Schullehner, K., Dick, R., Fiesselmann, A., and Gierl, A. 2009. Benzoxazinoid biosynthesis, a model for evolution of secondary metabolic pathways in plants. Phytochemistry 7015–16:1645–1651.
Grambow, H. J., Luckge, J., Klausener, A., and Muller, E. 1986. Occurrence of 2-(2-hydroxy-4,7-dimethoxy-2h-1,4-benzoxazin-3-one)-beta-d-glucopyranoside in Triticum aestivum leaves and its conversion into 6-methoxy-benzoxazolinone. Zeit. Für Natur. 417–8:684–690.
Gutierrez, C., Castanera, P., and Torres, V. 1988. Wound-induced changes in DIMBOA (2,4 dihydroxy-7-methoxy-2h-1,4 benzoxazin-3(4h)-one) concentration in maize plants caused by Sesamia nonagrioides (Lepidoptera: Noctuidae). Ann. App. Biol. 1133:447–454.
Halitschke, R. and Baldwin, I. T. 2003. Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant J. 366:794–807.
Hedin, P. A., Davis, F. M., and Williams, W. P. 1993. 2-Hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one (N-O-Me-DIMBOA), a possible toxic factor in corn to the Southwestern corn borer. J. Chem. Ecol. 193:531–542.
Howe, G. A. and Jander, G. 2008. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 59:41–66.
Huffaker, A., Dafoe, N. J., and Schmelz, E. A. 2011. ZmPep1, an ortholog of Arabidopsis Elicitor Peptide 1, regulates maize innate immunity and enhances disease resistance. Plant Physiol. 1553:1325–1338.
Kojima, W., Fujii, T., Suwa, M., Miyazawa, M., and Ishikawa, Y. 2010. Physiological adaptation of the Asian corn borer Ostrinia furnacalis to chemical defenses of its host plant, maize. J. Insect Physiol. 569:1349–1355.
Koornneef, A. and Pieterse, C. M. J. 2008. Cross talk in defense signaling. Plant Physiol. 1463:839–844.
Livak, K. J. and Schmittgen, T. D. 2001. Analysis of relative gene expression data using real time quantitative PCR and the 2 −∆∆CT method. Methods 254:402–408.
Mao, J. Q., Burt, A. J., Ramputh, A. I., Simmonds, J., Cass, L., Hubbard, K., Miller, S., Altosaar, I., and Arnason, J. T. 2007. Diverted secondary metabolism and improved resistance to European corn borer (Ostrinia nubilalis) in maize (Zea mays L.) transformed with wheat oxalate oxidase. J. Agric. Food Chem. 557:2582–2589.
McMullen, M. D., Frey, M., and Degenhardt, J. 2009. Genetics and biochemistry of insect resistance in maize. Handbook of Maize: Its Biology:271–289.
Niemeyer, H. M. 2009. Hydroxamic acids derived from 2-hydroxy-2h-1,4-benzoxazin-3(4h)-one: key defense chemicals of cereals. J. Agric. Food Chem. 575:1677–1696.
Oikawa, A., Ishihara, A., Hasegawa, M., Kodama, O., and Iwamura, H. 2001. Induced accumulation of 2-hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one glucoside (HDMBOA-Glc) in maize leaves. Phytochemistry 567:669–675.
Oikawa, A., Ishihara, A., and Iwamura, H. 2002. Induction of HDMBOA-Glc accumulation and DIMBOA-Glc 4-o-methyltransferase by jasmonic acid in Poaceous plants. Phytochemistry 613:331–337.
Oikawa, A., Ishihara, A., Tanaka, C., Mori, N., Tsuda, M., and Iwamura, H. 2004. Accumulation of HDMBOA-Glc is induced by biotic stresses prior to the release of MBOA in maize leaves. Phytochemistry 6522:2995–3001.
Ostlie, K., Hutchinson, W., and Hellmich, R. 1997. Bt Corn and the European Corn Borer. St. Paul, MN: University of Minnesota.
Saniewski, M., Ueda, J., and Miyamoto, K. 2002. Relationships between jasmonates and auxin in regulation of some physiological processes in higher plants. Acta Physiol. Planta. 242:211–220.
Sasai, H., Ishida, M., Murakami, K., Tadokoro, N., Ishihara, A., Nishida, R., and Mori, N. 2009. Species-specific glucosylation of DIMBOA in larvae of the rice armyworm. Biosci. Biotech. and Biochem. 736:1333–1338.
Schmelz, E. A., Alborn, H. T., and Tumlinson, J. H. 2003a. Synergistic interactions between volicitin, jasmonic acid and ethylene mediate insect-induced volatile emission in Zea mays. Physiol. Plant. 1173:403–412.
Schmelz, E. A., Engelberth, J., Alborn, H. T., O’Donnell, P., Sammons, M., Toshima, H., and Tumlinson, J. H. 2003b. Simultaneous analysis of phytohormones, phytotoxins, and volatile organic compounds in plants. Proc. Natl. Acad. Sci. USA 10018:10552–10557.
Schmelz, E. A., Engelberth, J., Tumlinson, J. H., Block, A., and Alborn, H. T. 2004. The use of vapor phase extraction in metabolic profiling of phytohormones and other metabolites. Plant J. 395:790–808.
Schmelz, E. A., Kaplan, F., Huffaker, A., Dafoe, N. J., Vaughan, M. M., Ni, X. Z., Rocca, J. R., Alborn, H. T., and Teal, P. E. 2011. Identity, regulation, and activity of inducible diterpenoid phytoalexins in maize. Proc. Natl. Acad. Sci. USA 10813:5455–5460.
Scott, I. M., Thaler, J. S., and Scott, J. G. 2010. Response of a generalist herbivore Trichoplusia ni to jasmonate-mediated induced defense in tomato. J. Chem. Ecol. 365:490–499.
Stout, M. J., Riggio, M. R., and Yang, Y. 2009. Direct induced resistance in Oryza sativa to Spodoptera frugiperda. Environ. Entomol. 384:1174–1181.
Thornburg, R. W. and Li, X. Y. 1991. Wounding Nicotiana tabacum leaves causes a decline in endogenous indole-3-acetic acid. Plant Physiol. 963:802–805.
Wasternack, C. and Kombrink, E. 2010. Jasmonates: structural requirements for lipid-derived signals active in plant stress responses and development. ACS Chem. Biol. 51:63–77.
Woodward, M. D., Corcuera, L. J., Helgeson, J. P., and Upper, C. D. 1978. Decomposition of 2,4-dihydroxy-7-methoxy-2h-1,4-benzoxazin-3(4h)-one in aqueous solutions. Plant Physiol. 615:796–802.
ZAR, J. 1999. Biostatistical Analysis. Prentice-Hall,Upper Saddle River, NJ.
Acknowledgements
We thank B. Forguson for greenhouse assistance, H. Alborn, and S. Willms for technical assistance, and student workers M. Legaspi and J. Thomas for laboratory assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Validation plots evaluating target and reference gene primer efficiency for quantitative real-time PCR. The variation of ΔCT (CT target–CT reference) with template cDNA dilutions (1, 1:10, 1:100, 1:1000) was determined to verify that amplification reactions had the same PCR efficiency. The slope of the resulting semi-log regression line was used as criterion for passing the validation experiment. The absolute value of the slope of ΔCT vs. log input for each primer pair was <0.1. A) anthranilate synthase subunit 2 ASsub2, B) anther ear 2 An2 and C) allene oxide synthase AOS primers were validated with elongation factor 1α EF1α and D) 1-aminocyclopropane-1-carboxylate oxidase ACC Ox was validated with ribosomal protein 17 RPL17. (PDF 31 kb)
Supplemental Fig. 2
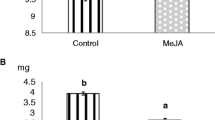
Effect of jasmonic acid (JA) and ethylene (ET) precursor, aminocyclopropane-1-carboxylic acid (ACC) on kauralexin accumulation. Comparison of average total kauralexin concentrations at 24 h between control, wounded, JA, ACC and JA and ACC treated stem internode tissue (N = 5, ±SEM). Different letters (a–c) represent significant differences (P < 0.01 for all ANOVAS; P < 0.05 for Tukey test corrections for multiple comparisons). (PDF 12 kb)
Rights and permissions
About this article
Cite this article
Dafoe, N.J., Huffaker, A., Vaughan, M.M. et al. Rapidly Induced Chemical Defenses in Maize Stems and Their Effects on Short-term Growth of Ostrinia nubilalis . J Chem Ecol 37, 984–991 (2011). https://doi.org/10.1007/s10886-011-0002-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-011-0002-9