Abstract
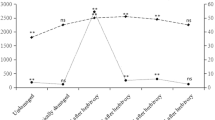
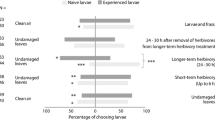
Neonate fall armyworms [FAW; Spodoptera frugiperda (Smith)] often encounter conspecific herbivore damage as they disperse from an egg mass to an initial feeding site. We investigated the orientation responses of dispersing neonates to herbivore damage in cowpea seedlings, specifically examining whether neonate behaviors were affected by inceptin, the primary elicitor of FAW-induced defenses in cowpea leaves. We focused on responses to damage caused by conspecific first instars, as might occur during the dispersal of siblings from an egg mass. Inceptin contents of damaging first instar FAW were controlled through their diets, with leaf-fed FAW producing inceptins in their oral secretions, and root-fed or starved FAW lacking these elicitors. In a bioassay designed to evaluate neonate dispersal off a host plant, a higher percentage of neonates remained on herbivore-induced or inceptin-treated plants than on undamaged plants, mechanically damaged plants, freshly damaged plants, or on plants damaged by FAW lacking inceptins. Further investigations of neonate responses to plant odors with a four-arm olfactometer demonstrated that neonate attraction to odors from 4-h old FAW damage was strongly dependent on previous diet of the damaging larvae. Neonates were attracted to odors from 4-h old FAW damage over odors from undamaged plants or fresh FAW damage, provided that the damaging larvae had previously ingested leaf material. In a direct comparison of odors from induced plants, plants damaged by leaf-fed FAW were as attractive as plants treated with synthetic inceptin. GC-MS analysis confirmed that (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT) was the major volatile induced by FAW herbivory. While both DMNT and undamaged plant odors were more attractive than air, neonates preferred DMNT-supplemented plant odors. These results suggest that neonate FAW exploit herbivore-induced plant volatiles as host plant location and recognition cues.






Similar content being viewed by others
References
Anderson, P., Hilker, M., and Löfqvist, J. 1995. Larval diet influence on oviposition behavior in Spodoptera littoralis. Entomol. Exp. Appl 74:71–82.
Bernasconi, M. L., Turlings, T. C. J., Ambrosetti, L., Bassetti, P., and Dorn, S. 1998. Herbivore-induced emissions of maize volatiles repel the corn leaf aphid, Rhopalosiphum maidis. Entomol. Exp. Appl 87:133–142.
Bernays, E. A., Singer, M. S., and odrigues, D. 2004. Foraging in nature: foraging efficiency and attentiveness in caterpillars with different diet breadths. Ecol. Entomol 29:389–397.
Bolter, C. J., Dicke, M., Vanloon, J. J. A., Visser, J. H., and Posthumus, M. A. 1997. Attraction of Colorado potato beetle to herbivore-damaged plants during herbivory and after its termination. J. Chem. Ecol 23:1003–1023.
Carroll, M. J., Schmelz, E. A., Meagher, R. L., and Teal, P. E. A. 2006. Attraction of Spodoptera frugiperda larvae to volatiles from herbivore-damaged maize seedlings. J. Chem. Ecol 32:1911–1924.
Carlsson, M. A., Anderson, P., Hartlieb, E., and Hansson, B. S. 1999. Experience-dependent modification of orientational response to olfactory cues in larvae of Spodoptera littoralis. J. Chem. Ecol 25:2445–2454.
Chapman, J. W., Williams, T., Escribano, A., Caballero, P., Cave, R. D., and Goulson, D. 1999. Fitness consequences of cannibalism in the fall armyworm, Spodoptera frugiperda. Behav. Ecol 10:298–303.
Colazza, S., Fucarino, A., Peri, E., Salerno, G., Conti, E., and Bin, F. 2004. Insect oviposition induces volatile emission in herbaceous plants that attracts egg parasitoids. J. Exp. Biol 207:47–53.
D’alessandro, M., Held, M., Triponec, Y., and Turlings, T. C. J. 2006. The role of indole and other shikimic acid derived maize volatiles in the attraction of two parasitic wasps. J. Chem. Ecol 32:2733–2748.
D’alessandro, M., and Turlings, T. C. J. 2005. In situ modification of herbivore-induced plant odors: A novel approach to study the attractiveness of volatile organic compounds to parasitic wasps. Chem. Senses 30:739–753.
Davis, F. M., Williams, W. P., Chang, Y. M., Baker, G. T., and Hedin, P. A. 1999. Differential growth of fall armyworm larvae (Lepidoptera: Noctuidae) reared on three phenotypic regions of whorl leaves from a resistant and a susceptible maize hybrid. Fla. Entomol 82:248–254.
De Moraes, C. M., Mescher, M. C., and Tumlinson, J. H. 2001. Caterpillar-induced nocturnal plant volatiles repel nonspecific females. Nature 410:577–580.
Del Campo, M. L., Miles, C. I., Schroeder, F. C., Mueller, C., Booker, R., and enwick, J. A. 2001. Host recognition by the tobacco hornworm is mediated by a host plant compound. Nature 411:186–189.
Dicke, M., Gols, R., Ludeking, D., and Posthumus, M. A. 1999. Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. J. Chem. Ecol 25:1907–1922.
Dicke, M., and Sabelis, M. W. 1988. How plants obtain predatory mites as bodyguards. Neth. J. Zool 38:148–165.
Dicke, M., Van Poecke, R. M. P., and De Boer, J. G. 2003. Inducible indirect defence of plants: from mechanisms to ecological functions. Bas. Appl. Ecol 4:27–42.
Dicke, M., and Vet, L. E. M. 1999. Plant-carnivore interactions: evolutionary and ecological consequences for plant, herbivore and carnivore, pp. 483–520, in H. Olff, V. K. Brown, and R. H. Drent (eds.). Herbivores: Between Plants and PredatorsOxford, Blackwell Science Oxford.
Doak, P. 2000. Population consequences of restricted dispersal for an insect herbivore in a subdivided habitat. Ecology 81:1828–1841.
Gouinguené, S., Alborn, H., and Turlings, T. C. J. 2003. Induction of volatile emissions in maize by different larval instars of Spodoptera littoralis. J. Chem. Ecol 29:145–162.
Gouinguené, S., Pickett, J. A., Wadhams, L. J., Birkett, M. A., and Turlings, T. C. J. 2005. Antennal electrophysiological responses of three parasitic wasps to caterpillar-induced volatiles from maize (Zea mays mays), cotton (Gossypium herbaceum), and cowpea (Vigna unguiculata). J. Chem. Ecol 31:1023–1038.
Harris, M. O., Sandanayake, M., and Foster, S. P. 1999. Chemical stimuli from apple influence the behavior of neonate caterpillars of the generalist herbivore, Epiphyas postvittana. J. Chem. Ecol 25:1717–1738.
Heath, R. R., and Manukian, A. 1994. An automated-system for use in collecting volatile chemicals released from plants. J. Chem. Ecol 20:593–608.
Heath, R. R., and Sonnet, P. E. 1980. Technique for in situ coating of Ag+ onto silica gel in HPLC columns for the separation of geometrical isomers. J. Liquid Chromatogr 3:1129–1135.
Hilker, M., and Klein, B. 1989. Investigation of oviposition deterrent in larval frass of Spodoptera littoralis (Boisd.). J. Chem. Ecol 15:929–938.
Hoballah, M. E., Kollner, T. G., Degenhardt, J., and Turlings, T. C. J. 2004. Costs of induced volatile production in maize. Oikos 105:168–180.
Hoballah, M. E., and Turlings, T. C. J. 2005. The role of fresh versus old leaf damage in the attraction of parasitic wasps to herbivore-induced maize volatiles. J. Chem. Ecol 31:2003–2018.
Hoballah, M. E. F., Tamo, C., and Turlings, T. C. J. 2002. Differential attractiveness of induced odors emitted by eight maize varieties for the parasitoid Cotesia marginiventris: Is quality or quantity important? J. Chem. Ecol 28:951–968.
Kessler, A., and Baldwin, I. T. 2001. Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144.
Kessler, A., and Baldwin, I. T. 2002. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol 53:299–328.
Landolt, P. J. 1993. Effects of host plant leaf damage on cabbage moth attraction and oviposition. Entomol. Exp. Appl 67:79–85.
Landolt, P. J., Brumley, J. A., Smithhisler, C. L., Biddick, L. L., and Hofstetter, R. W. 2000. Apple fruit infested with codling moth are more attractive to neonate codling moth larvae and possess increased amounts of (E,E)-alpha-farnesene. J. Chem. Ecol 26:1685–1699.
Luginbill, P. 1928. The fall armyworm. U.S. Department of Agriculture Technical Bulletin 34:1–91.
Meagher, R. L., Nagoshi, R. N., Stuhl, C., M, and E. R. 2004. Larval development of fall armyworm (Lepidoptera: Noctuidae) on different cover crop plants. Fla. Entomol 87:454–460.
Meiners, T., and Hilker, M. 1997. Host location in Oomyzus gallerucae (Hymenoptera: Eulophidae), an egg parasitoid of the elm leaf beetle Xanthogaleruca luteola (Coleoptera, Chrysomelidae). Oecologia 112:87–93.
Meiners, T., and Hilker, M. 2000. Induction of plant synomones by oviposition of a phytophagous insect. J. Chem. Ecol 26:221–232.
Paré, P. W., Farag, M. A., Krishnamachari, V., Zhang, H. M., yu, C. M., and Kloepper, J. W. 2005. Elicitors and priming agents initiate plant defense responses. Photosynth. Res 85:149–159.
Paré, P. W., and Tumlinson, J. H. 1997. Induced synthesis of plant volatiles. Nature 385:30–31.
Paré, P. W., and Tumlinson, J. H. 1999. Plant volatiles as a defense against insect herbivores. Plant Physiol 121:325–331.
Pickett, J. A., Bruce, T. J. A., Chamberlain, K., Hassannali, A., Khan, Z. R., Matthes, M. C., Napier, J. A., Smart, L. E., Wadhams, L. J., and Woodcock, C. M. 2006. Plant volatiles yielding new ways to exploit plant defence, pp. 161–173, in M. Dicke, and W. Takken (eds.). Chemical Ecology: from Gene to EcosystemSpringer, Wageningen, The Netherlands.
Pitre, H. N., Mulrooney, J. E., and Hogg, D. B. 1983. Fall armyworm (Lepidoptera: Noctuidae) oviposition: crop preferences and egg distribution on plants. J. Econ. Entomol 76:463–466.
Prokopy, R. J., and Roitberg, B. D. 2001. Joining and avoidance behavior in nonsocial insects. Annu. Rev. Entomol 46:631–665.
Renwick, J. A. A. 2001. Variable diets and changing taste in plant-insect relationships. J. Chem. Ecol 27:1063–1076.
Roitberg, B. D., and Mangel, M. 1993. Parent-offspring conflict and life history consequences in herbivorous insects. Am. Nat 1442:443–456.
Rott, A. S., Hackermann, J., Brand, N., Vallat, A., and Dorn, S. 2005. Parasitoid exploitation of the seasonal variation in host plant volatile emission for herbivore location. Entomol. Exp. Appl 115:199–205.
Schmelz, E. A., Alborn, H. T., and Tumlinson, J. H. 2001. The influence of intact-plant and excised-leaf bioassay designs on volicitin- and jasmonic acid-induced sesquiterpene volatile release in Zea mays. Planta 214:171–179.
Schmelz, E. A., Carroll, M. J., Leclere, S., Phipps, S. M., Meredith, J., Chourey, P. S., Alborn, H. T., and Teal, P. E. A. 2006. Fragments of ATP synthase mediate plant perception of insect attack. Proc. Natl. Acad. Sci. U.S.A 103:8894–8899.
Schoonhoven, L. M., and Van Loon, J. J. A. 2002. An inventory of taste in caterpillars: Each species its own key. Acta Zool. Acad. Sci. H 48:215–263.
Shiojiri, K., Ozawa, R., and Takabayashi, J. 2006a. Plant volatiles, rather than light, determine the nocturnal behavior of a caterpillar. Plos Biology 4:1044–1047.
Shiojiri, K., Kishimoto, K., Ozawa, R., Kugimiya, S., Urashimo, S., Arimura, G., Horiuchi, J., Nishioka, T., Matsui, K., and Takabayashi, J. 2006b. Changing green leaf volatile biosynthesis in plants: An approach for improving plant resistance against both herbivores and pathogens. Proc. Natl. Acad. Sci. U.S.A 103:16672–16676.
Sparks, A. N. 1979. A review of the biology of the fall armyworm. Fla. Entomol 62:82–86.
Stamps, J., and Krishnan, V. V. 2005. Nonintuitive cue use in habitat selection. Ecology 86:2860–2867.
Suazo, A., Torto, B., Teal, P. E. A., and Tumlinson, J. H. 2003. Response of the small hive beetle (Aethina tumida) to honey bee (Apis mellifera) and beehive-produced volatiles. Apidologie 34:525–533.
Thompson, J. N. 1988. Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol. Exp. Appl 47:3–14.
Turlings, T. C. J., Alborn, H. T., Loughrin, J. H., and Tumlinson, J. H. 2000. Volicitin, an elicitor of maize volatiles in oral secretion of Spodoptera exigua: Isolation and bioactivity. J. Chem. Ecol 26:189–202.
Turlings, T. C. J., Lengwiler, U. B., Bernasconi, M. L., and Wechsler, D. 1998. Timing of induced volatile emissions in maize seedlings. Planta 207:146–152.
Turlings, T. C. J., Loughrin, J. H., Mccall, P. J., ose, U. S., Lewis, W. J., and Tumlinson, J. H. 1995. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc. Natl. Acad. Sci. U.S.A 92:4169–4174.
Turlings, T. C. J., Tumlinson, J. H., and Lewis, W. J. 1990. Exploitation of herbivore-induced plant odors by host-seeking wasps. Science 250:1251–1253.
Ward, S. A. 1987. Optimal habitat selection in time-limited dispersers. Am. Nat 129:568–579.
Weatherston, I., Miller, D., and Dohse, L. 1985. Capillaries as controlled-release devices for insect pheromones and other volatile substances - a reevaluation. Part I. Kinetics and development of predictive model for glass capillaries. J. Chem. Ecol 11:953–965.
Yang, G., Wiseman, B. R., and Espelie, K. E. 1993. Movement of neonate fall armyworm (Lepidoptera: Noctuidae) larvae on resistant and susceptible genotypes of corn. Environ. Entomol 22:547–553.
Zalucki, M. P., Clarke, A. R., and Malcolm, S. B. 2002. Ecology and behavior of first instar larval Lepidoptera. Annu. Rev. Entomol 47:361–393.
Zangerl, A. R. 2003. Evolution of induced plant responses to herbivores. Bas. Appl. Ecol 4:91–103.
Zangerl, A. R., and Berenbaum, M. R. 1992. Oviposition patterns and hostplant suitability - parsnip webworms and wild parsnip. Am. Midl. Nat 128:292–298.
Acknowledgment
We thank Sean Collins and Art Zangerl for comments that improved the manuscript. We especially thank Rob Meagher, Charlie Dillard, and Nancy Lowman for providing the insects used in these experiments. We also thank Julia Meredith and Mary Searle for assistance in the maintenance of plants used in this experiment.
Author information
Authors and Affiliations
Corresponding author
Additional information
The use of trade, firm, or corporation names in this publication (or page) is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the United States Department of Agriculture or the Agricultural Research Service of any product or service to the exclusion of others that may be suitable.
Rights and permissions
About this article
Cite this article
Carroll, M.J., Schmelz, E.A. & Teal, P.E.A. The Attraction of Spodoptera frugiperda Neonates to Cowpea Seedlings is Mediated by Volatiles Induced by Conspecific Herbivory and the Elicitor Inceptin. J Chem Ecol 34, 291–300 (2008). https://doi.org/10.1007/s10886-007-9414-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9414-y