Abstract
The effects of induced plant responses on herbivores are categorised as direct, by reducing herbivore development, or indirect, by affecting the performance of natural enemies. Here, we investigated a tritrophic system, which included the herbivore Heortia vitessoides, its host plant Aquilaria sinensis, and its predator Cantheconidea concinna. Herbivore-damaged A. sinensis plants released significantly greater amounts of volatiles than undamaged and mechanically damaged plants, with an obvious temporal trend. One day after initial herbivore damage, A. sinensis plants released large amounts of volatile compounds. Volatile compounds release gradually decreased over the next 3 d. The composition and relative concentrations of the electroantennographic detection (EAD)-active compounds, emitted after herbivore damage, varied significantly over the 4-d measurement period. In wind tunnel bioassays, mated H. vitessoides females showed a preference for undamaged plants over herbivore and mechanically damaged A. sinensis plants. In Y-tube bioassays, C. concinna preferred odours from herbivore-damaged plants to those from undamaged plants, especially after the early stages of insect attack. Our results indicate that the herbivore-induced compounds produced in response to attack by H. vitessoides larvae on A. sinensis plants could be used by both the herbivores themselves and their natural enemies to locate suitable host plants and prey, respectively.
Similar content being viewed by others
Introduction
Plants have developed various adaptive and defensive strategies against insect herbivory over the course of their long evolutionary history1. Intact, healthy plants normally release various volatile organic compounds (VOCs), which act as important signals for herbivores to locate host plants for oviposition and feeding2,3,4. Plants under herbivore attack respond by emitting much more diverse volatiles in greater quantities compared to healthy, undamaged plants. The diverse volatiles released in response to herbivore attack are termed herbivore induced plant volatiles (HIPVs)5,6,7. HIPVs are more likely to be detected by herbivores, their natural enemies, and neighbouring plants compared to the VOCs released by healthy plants, because of the particular chemical constituents6. HIPVs may mediate tritrophic interactions between plants, herbivores, and natural enemies of herbivores, and perhaps even interactions at a fourth trophic level, with hyperparasitoids8,9,10.
Aquilaria sinensis (Lour.) Gilg (Thymelaeaceae) is an economically important evergreen tree native to China, which grows mainly in tropical climates, including the provinces of Hainan, Guangdong, Guangxi, Fujian, Yunnan, and Taiwan. A. sinensis is the principal source of Chinese agarwood, a resinous A. sinensis heartwood formed in response to fungal infection. In China, agarwood is used in religious ceremonies, traditional medicine, and as incense11.
Heortia vitessoides Moore (Lepidoptera: Crambidae) is the most destructive insect pest of A. sinensis throughout the tree’s range in southern China12; the hosts of H. vitessoides include several species of the genera Aquilaria and Rhus13,14. In China, the larvae feed solely on the leaves of A. sinensis12,13,14. Large infestations of these caterpillars have defoliated large areas of forest in southern China and caused significant economic losses. Apart from the heavy use of pesticides, there is no known effective method for controlling this pest.
Su13 suggested that the young leaves of A. sinensis were the sole emitters of VOCs attractive to H. vitessoides females seeking oviposition sites. We previously identified and compared VOCs from young and old A. sinensis leaves that potentially attract H. vitessoides. We also tested the behavioural responses of H. vitessoides to synthetic blends of these VOCs in wind tunnel and field tests, and established a relationship between leaf age preference and host plant recognition in H. vitessoides12. We found qualitative and quantitative differences between the odour profiles of young and old leaves. Wind tunnel and field tests confirmed that a nine-component mixture based on young leaves (comprised of hexanal, limonene, 2-hexanol, octanal, (Z)-3-hexenyl acetate, (Z)-3-hexen-1-ol, nonanal, decanal, and 2,6,10-trimethyl-dodecane at a ratio of 2:16:9:4:63:100:13:10:5) attracted significantly more moths than the three component mixture based on old leaves (comprised of nonanal, decanal, and 2,6,10-trimethyl-dodecane in a ratio of 11:14:26). The volatile signals from young A. sinensis leaves allowed H. vitessoides females to discriminate suitable larval hosts from the background chemical environment, and guided orientation of flights towards these plants for oviposition.
In a more recent study, we found that female adult oviposition on young A. sinensis leaves was reduced in response to damage caused by H. vitessoides larvae. In other words, female adults preferred to lay eggs on the healthy, intact young leaves. In addition, many natural enemies of H. vitessoides larvae, including Cantheconidea concinna, are found on herbivore-damaged A. sinensis plants15. To date many studies have shown that HIPVs can either attract or repel the same or different species of herbivores16,17, and even attract their natural enemies5. For instance, Tetranycbus evansi adults were more attracted to plants attacked by conspecific larvae than to undamaged plants in olfactometer experiments18. Caterpillar-induced nocturnal tobacco plant volatiles were found to repel ovipositing conspecific moths16. Kappers et al.19 suggested that HIPVs play a very important role in plant defences against herbivores, both directly and indirectly, as cues that attract predatory and parasitic natural enemies of herbivores.
Here, we hypothesise that HIPVs emitted by A. sinensis significantly reduce herbivore oviposition and increase recruitment of their natural enemies, and ask: How do the moth H. vitessoides and its predatory enemy C. concinna respond to HIPV emissions from A. sinensis? We aimed to (1) identify and compare VOCs released by undamaged, mechanically damaged, and herbivore-damaged A. sinensis plants; (2) analyse the antennal and behavioural responses of mated H. vitessoides females to these volatile compounds; and (3) examine whether HIPVs emitted by A. sinensis affect the host-searching behaviour of C. concinna on a host-infested plant. In this study, we sought to elucidate the role of HIPVs emitted from herbivore-damaged plants and improve our understanding of how herbivore insects locate hosts and are located by their predators in a tree ecosystem.
Results
The concentrations and compositions of the VOCs, which belonged to eight groups: alcohol, aldehyde, hydrocarbon, ketone, ester, benzenoid, terpenoid, and green leaf volatile, differed significantly among treatments (Table 1). The volatile blends emitted by undamaged, mechanically damaged, and herbivore-damaged A. sinensis plants were significantly different, both quantitatively (F = 1315.532, DF = 5, P < 0.001) and to a lesser degree qualitatively (F = 288.600, DF = 5, P < 0.001) (Table 1 and Fig. 1).
Concentration and number of all volatile compounds detected (±SE) from headspace collections from undamaged, mechanically damaged, and herbivore-damaged Aquilaria sinensis plants damaged by leaf-feeding larvae of Heortia vitessoides over a 4-d period. N = 6 for each treatment group. P-values based on one-way ANOVAs conducted at each treatment: *P < 0.05; **P < 0.01; ns = P ≥ 0.05.
Identification of plant volatiles quantitatively
The total absolute volatile emissions (taken as the sum of the concentration of individual volatile compounds) (Fig. 1) and relative percentages of different VOC classes from undamaged (Fig. 2A) and mechanically damaged plants (Fig. 2B) were similar. However, VOCs, collected after the 8 hr initial caterpillar damage, varied significantly compared to the other two groups, and increased almost linearly over time, until sampling days 2–4 when the VOCs decreased sharply (Fig. 1). Specifically, in the VOC profile of 1-day herbivore-damaged plants, the release of two classes of VOCs, green leaf volatiles (GLVs) and terpenoids (we named them increased group, IG), were obviously increased, which made up the bulk of VOCs after the initial 8 hr of caterpillar damage (Fig. 2C). The increase in 1-day VOC concentration could be attributed to 6 GLVs [3-hexanol, (Z)-3-hexen-1-ol, (E)-2-hexen-1-ol, (Z)-3-hexenyl acetate, 2-hexen-1-ol, acetate, and 3-hexanone] and 6 terpenoids [β-myrcene, (E)-β-ocimene, (Z)-β-ocimene, linalool, caryophyllene, and α-farnesene] (Table 1). Of these, (Z)-β-ocimene (F = 228.340, DF = 5, P < 0.001) and 2 GLVs [(Z)-3-hexen-1-ol (F = 522.61, DF = 5, P < 0.001) and (Z)-3-hexenyl acetate (F = 57.25, DF = 5, P < 0.001)] were predominant in all treatments, and showed significantly higher amounts after the initial 8 hr of caterpillar damage (treatment 3) compared to the other treatments (Table 1). The absolute volatile emissions of hydrocarbons also increased after herbivory initial damage. However, the other five classes of VOCs, alcohols, aldehydes, ketones, esters, and benzenoids (we named as decreased group, DG), decreased after the initial 8 hr of caterpillar damage (Fig. 2C). Of these, total emissions of 2 aldehydes [nonanal and decanal] and 1 ketone [6-methyl-5-hepten-2-one] significantly decreased (F = 23.62, DF = 5, P < 0.001 for nonanal; F = 225.21, DF = 5, P < 0.001 for decanal; F = 26.66, DF = 5, P < 0.001 for 6-methyl-5-hepten-2-one) (Table 1).
In contrast to the 1-day group, in the VOC profiles of 2- to 3-day herbivore-damaged plants, the increased groups (IG) decreased or disappeared (Fig. 2D,E). Of these, several compounds obviously decreased, including 3 GLVs [(Z)-3-hexen-1-ol, (E)-2-hexen-1-ol, and (Z)-3-hexenyl acetate (F = 522.61, 640.41, 57.25, respectively, DF = 5, P < 0.001)] and 4 terpenoids [(E)-β-ocimene, (Z)-β-ocimene, linalool, and α-farnesene (F = 64.12, 228.34, 533.58, 253.00, respectively, DF = 5, P < 0.001)] (Table 1). However, the decreased groups (DG) increased. Specifically, 1 alcohol [2-decen-1-ol], 3 aldehydes [octanal, nonanal, and decanal], and 1 ketone [6-methyl-5-hepten-2-one] obviously increased (Table 1).
The variation trend was further developed on the fourth day after the initial herbivore-damage (treatment 6), when extremely low amounts of increased groups (IG) (GLVs and terpenoids) were detected, accompanied with higher amounts of decreased groups (DG) (alcohols, aldehydes, ketones, esters, and benzenoids) (Table 1, Fig. 2F).
Identification of plant volatiles qualitatively
The absolute numbers (Fig. 1) and relative percentages of different VOC classes between undamaged (Fig. 3A) and mechanically damaged plants (Fig. 3B) were similar. However, the number of VOCs gradually increased in the herbivore-damaged plants (Fig. 1). Specifically, for VOC profiles of 1-day herbivore-damaged plants, the number of GLVs, terpenoids, and alcohols increased after the initial 8 hr of caterpillar damage. In comparison, the other VOC classes varied to a lesser degree after the initial caterpillar attack (Fig. 3C). Of the VOCs with increased variations, 6 GLVs [3-hexanol, (Z)-3-hexen-1-ol, (E)-2-hexen-1-ol, 3-hexanone, (Z)-3-hexenyl acetate, and 2-hexen-1-ol, acetate], 4 terpenoids [β-myrcene, linalool, epiglobulol, and caryophyllene], 3 alcohols [3-methyl-4-heptanol, 1-octen-3-ol, and 2-pentadecyn-1-ol] and 5 esters [butyl isobutyrate, heptyl butanoate, hexyl acetate, methyl benzoate, and ethyl hexadecanoate] emerged after the initial caterpillar attack, which were completely absent in undamaged plants. In contrast, 1 alcohol [2-decen-1-ol], and 1 aldehyde [octanal] disappeared after the initial 8 hr of caterpillar damage (Table 1).
Those VOCs which emerged during the initial damage disappeared; in particular, GLVs gradually decreased or disappeared during the subsequent 2- to 4- sampling dates (Fig. 3D,E,F). Of these, 4 GLVs [2-ethyl-1-hexanol, 3-hexanol, 3-hexanone, and 2-hexen-1-ol, acetate] and 2 terpenoids [β-myrcene, caryophyllene] disappeared in 2- to 4-day herbivore-damaged plants compared with the VOC profile of 1-day herbivore-damaged plants. However, 1 alcohol [2-decen-1-ol], and 1 aldehyde [octanal] came back during the subsequent sampling dates (Table 1). VOC profiles of 4-day herbivore-damaged plants (Fig. 3F) were almost similar to undamaged plants qualitatively (Fig. 3A).
PCA and hierarchical cluster analysis
Principal component analysis (PCA) clearly segregated the overall composition of the headspace volatile blends collected from the six plant treatments (Fig. 4). A scatter plot of the first and second principal components showed that principal component 1 was more discriminating than principal component 2. The two principal component axes accounted for 55.27% of the total variation in VOCs. The first PCA accounted for 37.50% and the second PCA accounted for 17.77%.
Scores plot of the principal component analysis (PCA) of headspace volatiles from undamaged, mechanically damaged, and herbivore-damaged Aquilaria sinensis plants damaged by leaf-feeding larvae of Heortia vitessoides over a 4-d period. Each single symbol (green circles) represents a sample. N = 6 for each treatment group. Black circles represent classification of these plants. The x-axis represents the first principal component (PC-1) and the y-axis represents the second principal component (PC-2), which accounted for 37.50% and 17.77% of the total variation, respectively.
PCA also segregated the volatile blends into three groups according to the behavioural effects on H. vitessoides females. Group A, comprised of the volatile blends detected in the undamaged and mechanically damaged A. sinensis plants, was the volatile blend that attracted female moths. Non-attractive blends clustered in two distinct groups: Group B1 was a volatile blend from 1 d after herbivory plant damage and Group B2 was comprised of blends from 2 d, 3 d, and 4 d after herbivory damage of A. sinensis plants. The volatile blend from 1d after herbivory damaged plants (Group B1), although unattractive, occupied a different position in the PCAs compared to the other three unattractive blends (Group B2) (Fig. 4). The number of compounds detected in this unattractive blend (Group B1) was exceptionally high, especially the terpenoids and green leaf volatiles (Table 1).
Hierarchical cluster analysis between-groups linkage was used to analyse the volatiles derived from six treatments, at a distance >5 and <20. They were divided into three clusters (Group A, B1, B2, Fig. 5). System clustering results were consistent with the PCA results.
Antennal responses of H. vitessoides to plant volatiles
In total, 10 compounds from the headspace of A. sinensis plants elicited antennal responses from H. vitessoides females: (1) (Z)-β-ocimene; (2) octanal; (3) nonanal; (4) 2-decen-1-ol; (5) decanal; (6) (Z)-3-hexenyl acetate; (7) hexyl acetate; (8) (Z)-3-hexen-1-ol; (9) 1-octen-3-ol; and (10) methyl benzoate. Of these, the terpenoid [(Z)-β-ocimene (peak 1)] and aldehydes [nonanal (peak 3) and decanal (peak 5)], common compounds among the six treatments, elicited consistent antennal responses (Fig. 6).
Simultaneously recorded GC-EAD using the antennae of Heortia vitessoides females in response to volatiles collected from undamaged (A), mechanically damaged (B), and herbivore (H. vitessoides) damaged Aquilaria sinensis plants 1 d (C), 2 d (D), 3 d (E), and 4 d (F) following insect attack. The upper trace represents the Flame Ionization Detector (FID) response and the lower trace represents the female-consistent EAD response. The EAD-active compounds were as follows: (1) (Z)-β-ocimene; (2) octanal; (3) nonanal; (4) 2-decen-1-ol; (5) decanal; (6) (Z)-3-hexenyl acetate; (7) hexyl acetate; (8) (Z)-3-hexen-1-ol; (9) 1-octen-3-ol; and (10) methyl benzoate.
Five compounds from the undamaged plants (treatment 1), including (Z)-β-ocimene (peak 1), octanal (peak 2), nonanal (peak 3), 2-decen-1-ol (peak 4), and decanal (peak 5), elicited antennal responses (Fig. 6A). Five compounds from mechanically damaged plants (treatment 2) elicited antennal responses; (Z)-3-hexenyl acetate (peak 6) was characteristic, while octanal (peak 2) was not characteristic of electroantennographic detection (EAD)-active compounds from mechanically damaged plants, compared to undamaged plants (Fig. 6B).
Compared to undamaged plants, both octanal (peak 2) and 2-decen-1-ol (peak 4) were not present in volatiles collected after herbivory initial damage (Fig. 6C). In addition, the other five characteristic VOCs, which emerged after the initial 8 hr of caterpillar damage and made up the bulk of VOCs in the sample date, also elicited antennal responses. These five VOCs included 2 GLVs [(Z)-3-hexenyl acetate (peak 6) and (Z)-3-hexen-1-ol (peak 8)], 1 ester [hexyl acetate (peak 7)], 1 alcohol [1-octen-3-ol (peak 9)], and 1 benzenoid [methyl benzoate (peak 10)] (Fig. 6C).
EAD-active profiles of A. sinensis plants on the second, third, and fourth day after the initial 8 hr herbivore-damage were similar (treatment 4–6) (Fig. 6D–F). The common compounds, (Z)-β-ocimene (peak 1), nonanal (peak 3), 2-decen-1-ol (peak 4), and decanal (peak 5), elicited consistent antennal responses. The five compounds, which emerged after the initial 8 hr damage, disappeared gradually. In particular, the EAD-active odour profiles of plants on the fourth day after the initial 8 hr herbivore-damage (treatment 6) were completely similar to undamaged plants (treatment 1) (Fig. 6A,F).
In summary, the antennae of H. vitessoides females responded to not only the most abundant compounds, such as (Z)-β-ocimene (peak 1), (Z)-3-hexenyl acetate (peak 6), and (Z)-3-hexen-1-ol (peak 8), but also to the less abundant compounds, such as octanal (peak 2), nonanal (peak 3), 2-decen-1-ol (peak 4), decanal (peak 5), hexyl acetate (peak 7), 1-octen-3-ol (peak 9), and methyl benzoate (peak 10) in all treatments (Fig. 6).
Wind tunnel bioassays
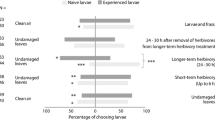
All six EAD-active blends that were tested stimulated H. vitessoides female upwind flights and approaches to within 5 cm of the source (Fig. 7). Synthetic blends mimicking undamaged plant VOCs (A) had the strongest attraction to females in the wind tunnel; 38.89% of females flew over 120 cm upwind, and 21.11% arrived within 5 cm of the source. The number of female upwind flights elicited by synthetic blend A was significantly higher than flights elicited by the other blends (F = 25.900, DF = 6, P < 0.001). Blend B, containing five compounds identified in the headspace of mechanically damaged plants, was the second most attractive to females. This blend resulted in 27.78% of females flying upwind and 16.67% approaching the source. Compared with blend A (mimicking undamaged plant VOCs) and blend B (containing five compounds identified in the headspace of mechanically damaged plants), the synthetic blends containing compounds released from herbivore-damaged A. sinensis plants (C, D, E, and F) elicited significantly fewer females upwind flights and landings near the source (F = 25.900, DF = 6, P < 0.001 for upwind flights; F = 15.127, DF = 6, P < 0.001 for landings near the source). There were no differences in the number of female upwind flights or landings near the source among the 4 blends from herbivore-damage plants (C, D, E, and F). The control (hexane solvent) did not induce any females to land near the source.
Number of responses of mated Heortia vitessoides females to synthetic blends from undamaged (A), mechanically damaged (B), herbivore (H. vitessoides) damaged Aquilaria sinensis plants 1 d (C), 2 d (D), 3 d (E), 4 d (F) following insect attack, and blank in the wind tunnel. Synthetic blends from different plant treatments eliciting consistent antennal responses in female H. vitessoides were prepared according to the natural ratios of each compound to the headspace collections (Table 2). Females were scored for upwind flights over 120 cm (white columns) and for approaching the source within 5 cm (black columns). Bars with the same colour and different letters were significantly different.
Y-tube bioassays
In the dual-choice bioassay, C. concinna, a predator of H. vitessoides larvae, preferred VOCs from herbivore-damaged plants to those from undamaged plants (Fig. 8). The predator was attracted by the odour of 1-day herbivore-damaged plants (X2 = 111.386, N = 30, P < 0.001), 2-day herbivore-damaged plants (X2 = 81.820, N = 30, P < 0.001), and 3-day herbivore-damaged plants (X2 = 50.000, N = 30, P < 0.001). The effect of 4-day herbivore-damaged plants odour was not significant (X2 = 5.120, N = 30, P = 0.034). Results from the one-way ANOVA showed that olfactory response rates of C. concinna to the odours from A. sinensis plants differed among damage treatments (F = 4.04, P < 0.05). C. concinna adults showed a preference for recently herbivore-damaged plants (1–3 d of herbivore damage) over plants with 4 d of damage.
Olfaction selection preference and responsiveness (i.e. percentage of females making a choice) of predatory Cantheconidea concinna to different treatments of Aquilaria sinensis plants in a Y-tube olfactometer. Undamaged plants were used as control. Treatment groups consisted of 1 d, 2 d, 3 d and 4 d mean herbivore (Heortia vitessoides) damaged A. sinensis plants. P-values are based on chi-square test: **P < 0.001, ns = P ≥ 0.05. Different lower-case letters on the left side of the bar indicate significant differences (one-way ANOVA followed by least significant difference’s multiple comparison test, P < 0.05).
Discussion
Plants commonly respond to herbivore attacks by releasing HIPVs6,20. The production and release of HIPVs can directly and indirectly affect herbivore performance and mediate interactions with other community members. Thus, HIPVs act as signals to herbivores, their natural enemies, and neighbouring plants6,8. These components have been well described in agricultural ecological systems, such as maize17, rape, and cotton21,22. Herein, we studied a tritrophic system which includes the herbivore H. vitessoides, its host tree A. sinensis, and its predator C. concinna. Our studies indicate that previously described tritrophic interactions in agricultural crop systems also apply to a forest ecosystem.
HIPVs may discourage oviposition of herbivores on damaged plants and may, therefore, be beneficial in reducing herbivore density23. Our behavioural bioassays showed that mated H. vitessoides females preferred VOC blends mimicking healthy, undamaged A. sinensis plants to those containing VOCs emitted by herbivore-damaged plants. This suggests that H. vitessoides females detect and assess VOCs released by A. sinensis to locate suitable oviposition sites and avoid the unsuitable sites. Similarly, in a dual-choice test weevils have been shown to prefer undamaged clover leaves to weevil-damaged leaves24. In behavioural bioassays, alate Aphis gossypii preferred the odour from undamaged cotton seedlings to that from A. gossypii-infested plants25. Recognition and the ability to locate suitable host plants using plant volatiles is beneficial for the offspring of insect herbivores20. Female insects generally prefer healthy, intact plants as oviposition sites, as these are more likely to provide newly hatched larvae with enough food resources. Thus, this strategy reduces the strength of intraspecific food competition, and increases individual survival and population growth26. In a previous study, we showed that mated H. vitessoides females were more attracted to young leaves than to old leaves of A. sinensis plants, and suggest that the former provides suitably tender food for freshly hatched, delicate young larvae12.
Many studies have shown that HIPVs may act as an important signal for natural enemies to locate their host/prey27,28. HIPVs are likely to act as important cues for natural enemies to locate damaged plants, and by extension, the herbivores attacking those plants, and, thus, may act as indirect plant defences23,29. Females laying eggs on undamaged plants also reduce the risk of parasitoid and predator attacks on larvae29. HIPVs are highly detectable and variable, and parasitoids and predators can distinguish these compounds to infer host suitability and even detect if hosts are parasitized or not6. HIPVs can increase predation and parasitism rates of herbivores, and, thus, reduce plant damage and increase reproductive output20. Our study demonstrates that C. concinna prefers the odours of herbivore-damaged plants to those from undamaged plants. Thus, HIPVs could be beneficial in attracting the predator C. concinna in response to insect attack.
Plants can produce various complex VOCs, which together make up the particular volatile spectrum of each species29,30. Some volatile compounds are continuously emitted, while many others are only released when plants are attacked by herbivores or mechanically damaged7. This damage response can play a key role in mediating multitrophic plant-insect interactions8,31. These complex volatile compounds resulting from damage tend to be released in greater variety and quantities than those from intact, healthy plants6,9. Numerous studies have shown both large quantitative increases and qualitative changes in VOC emissions as a result of mechanical and herbivore damage20,21. In particular, obvious differences were found between VOCs from herbivore-damaged A. sinensis plants compared to undamaged or mechanically damaged plants; herbivore damage elicited the release of a greater variety of VOCs, and in far greater quantities than mechanical damage. Herein, using mechanically-damaged plants as one of the treatments was valuable and allowed us to compare the difference between herbivore feeding and mechanical wounding to characterize a set of special HIPVs.
In general, the majority of HIPVs belong to green leaf volatiles (GLVs-C6 aldehydes, alcohols, and their esters), terpenoids, aromatics, and amino acid volatile derivatives32. Similarly, these classes detected in our study showed an increased emission pattern in response to herbivore feeding, most of which belonged to the increased groups (IG). However, HIPVs varied considerably over time in response to damage33. Different chemical classes have different change rhythms. Some GLVs are produced immediately after initial damage by the larvae of herbivores; the production and emission of these GLVs occurs almost instantaneously during the initial 1–2 hours after herbivore damage. While other chemicals, such as terpenoids, are released several hours after herbivore damage or the following day21,34; these HIPVs are synthetized de novo and emitted later33. Some studies have shown that there are different biosynthesis pathways, including autolytic oxidative breakdown of membrane fatty acids or nonmevalonate for different chemical classes.
All of the quantitative and qualitative changes in VOC emissions are always short-lasting. Once the damage ceases, the emission of these VOCs drops rapidly, making this a highly dynamic process21,22,35. In our study, the quantity and diversity of several HIPV classes, including GLVs or terpenoids, declined gradually soon after the attacks on the plant stopped. Simultaneously, several other classes, including aldehyde, alcohol, and ketone, almost disappeared after the initial damage, and recovered again during the subsequent 2–4 day sampling dates.
The rhythm of these changes in different chemical classes is consistent with the behaviour results in the wind tunnel. The increase in GLVs [(Z)-3-hexenyl acetate, (Z)-3-hexen-1-ol] and terpenoids [(Z)-β-ocimene] and the decrease in aldehydes [octanal, nonanal, and decanal] and alcohols [2-decen-1-ol] during the initial damage correspond to less attraction of H. vitessoides females to the synthetic blends that mimic the initial damaged plant VOCs. Conversely, the decrease of GLVs and terpenoids and the recovery of aldehydes and alcohols during the 2–4 day sampling dates correlated with the recovered attraction of females in the wind tunnel for synthetic blends that mimic the 2–4 day damaged plants. These data imply that HIPVs, including GLVs and terpenoids, are repellent for female H. vitessoides. However, some aldehydes and alcohols are attractive to female H. vitessoides. Most studies have found that adult moths are repelled by host volatiles released by conspecific larval feeding16,17,36, although this is by no means universal37. Clearly, more behavioural studies are needed to assess the impact of these various volatile components on insect behavior38.
Behavioural responses to VOCs from plants do not always mirror electrophysiology results in some insects. Not all GC-EAD active components are attractive in the behaviour assay and may act as a repellent. Wee et al.24 reported that lemon leaf volatiles elicited electrophysiological responses in weevils, but weevils were repelled by these compounds in behavioural bioassays. In our study, the three GC-EAD components [(Z)-3-hexenyl acetate, (Z)-3-hexen-1-ol, and (Z)-β-ocimene] were repellent in the wind tunnel assay. The amplitude of EAD is also not consistent to its behavioural activity. In our study, mated H. vitessoides females responded consistently and strongly to VOC blends mimicking headspace collections from herbivore-damaged A. sinensis plants in electroantennographic tests, but behavioural responses to these compounds in wind tunnel bioassays were weaker. Some studies have found discrepancies between electrophysiological and behavioural responses to VOCs in herbivores24,39. For example, the highest EAD responses from Pandemis heparana moths were obtained with the terpenes, linalool and DMNT, which are often key volatiles in herbivore deterrence36,40. The addition of the four compounds that elicited the smallest antennal responses resulted in improved levels of upwind flight of female grape berry moths (Paralobesia viteana)41.
Methods
Insects
H. vitessoides eggs were provided by Huazhou Green Life Co. Ltd (Guangdong, China) from the A. sinensis fields in the Chinese Medicinal Material Production Base (CMMPB). Newly hatched larvae were mass-reared for three instars in glass containers (diameter: 20 cm, height: 30 cm), and then separately transferred to smaller glass containers (diameter: 3 cm, height: 10 cm) with fresh A. sinensis leaves. Adults were provided with a 10% sugar-water solution on water-soaked cotton. All insects were reared in a climate-controlled room (25 ± 2 °C, 70 ± 5% RH, L16:D8).
Late-third to fourth-instar H. vitessoides larvae were used in the tests to induce the HIPVs. Larvae were starved overnight prior to all experiments to encourage active feeding immediately after being placed on plants. In behavioural assays, mated females were used. To obtain mated females, the newly emerged adult couples were placed in a cage (200 × 200 × 200 cm) with A. sinensis at a 2:1 ratio of male:female to ensure mating. Only females laying eggs were used in the wind tunnel bioassays. None of the females used in tests had previously been exposed to any of the tested odours and each was used only once42.
C. concinna nymphs, the primary predator of H. vitessoides larvae, were collected from the same fields as the original H. vitessoides eggs and reared in smaller glass containers (diameter: 3 cm, height: 10 cm) under the same conditions as their hosts. Late-third to fourth-instar H. vitessoides larvae were provided as food to C. concinna nymphs and adults. C. concinna adults used in Y-tube trials were 1–2 d old. All adults were starved overnight prior to trials and none had been exposed to any host plant or prey odour.
Plant materials and treatments
Healthy potted A. sinensis seedlings were cultivated in thin-meshed gauze cages (200 × 200 × 200 cm). No H. vitessoides damage occurred during cultivation and no insecticides or other specific treatments against H. vitessoides were used at the study site during the trials. The environmental condition in the thin-meshed gauze cages was set at a 16 hr light/8 hr dark photoperiod.
The tested A. sinensis seedlings were from the thin-meshed gauze cages. Plants that were about 70 cm tall were used in all experiments. Plants were individually wrapped in gauze mesh during the whole trials. These plants were randomly divided into three groups, including undamaged plants (treatment 1), plants cut using a razor (treatment 2), and plants infested with H. vitessoides larvae (treatment 3, 4, 5, 6). Throughout the experiment, all treatments were kept separate, to prevent possible plant-to-plant transmission of airborne signals.
Seven treatments were administered as follows. Treatment 1 (undamaged plants)-plants were enclosed by a fine-mesh gauze with enough space between the plant and gauze to protect from any insect herbivory and damage during the experiments. Treatment 2 (fresh mechanically damaged plants)-100 cuts (1 cm in length) were made on the leaves of each plant with a razor blade to simulate the damage caused by late-third to four-instar H. vitessoides larvae. Treatments 3–6 (H. vitessoides damaged plants)-100 late-third to four-instar H. vitessoides larvae were placed on each test plant and allowed to feed on the plant. Treatment 7 (clean bags)-odour samples were collected from clean roasting bags (40.6 × 44.4 cm; Reynolds roasting bag, Richmond, Virginia, USA) as a control.
For mechanical-damage treatment 2, the plants were immediately placed inside the volatile collection system (see below) after mechanical damage. Collections were performed for 8 h. For the herbivory treatments 3–6, each entire plant was individually wrapped in gauze mesh to prevent the larvae from escaping during the experiment. After 8 h of feeding, the gauze mesh and larvae were removed from the infested plant. Volatile collection experiments began after the removal of the larvae. Collections were conducted for 8 h every day for a successive 4 d period, corresponding to the four treatments, 3–6. Each collection was made at the same time (20:00–04:00) each day, corresponding to the oviposition peak period of H. vitessoides. The plants were weighed immediately after collection. Each treatment was repeated six times, with different plants, cut damage, and test larvae.
Plant volatile collections
We used a headspace collection system to collect headspace volatiles from plants. Living test plants were placed in a clean roasting bag. The bag was sealed around the plant stem with a self-sealing strip about 20 cm above soil-height34. Humidified, charcoal-filtered air was pulled through the bag with a pump (Beijing Institute of Labour Instruments, China) at 300 ml·min−1 and passed over an adsorbent cartridge. The adsorbent cartridge was a 0.5 × 10 cm glass column containing 50 mg of adsorbent (80/100 mesh, Supelco, Bellefonte, PA, USA). The Porapak Q (50 mg, 80–100 mesh, Supelco, Bellefonte, PA, USA) was held between plugs of glass wool. Each sample was aerated for 8 h. Volatiles were eluted from the adsorbent cartridge with 500 μl redistilled hexane at room temperature. An internal standard of 0.5 μg of benzaldehyde (99%, Fluka Production) was added to the extract for chemical quantification43. The final extracts were reduced to 50 μl using a slow stream of nitrogen and then subjected to gas chromatography mass spectrometry (GC-MS) and gas chromatography-electroantennographic detection (GC-EAD). If not used immediately, extracts were stored in glass vials at −18 °C until use.
GC-MS
Headspace extracts were analysed with an Agilent Technologies 6890 N gas chromatograph linked to a 5973 mass spectrometer (Palo Alto, CA, USA) with a polar DB-Wax or non-polar DB-5 fused silica column (both 30 m × 0.25 mm × 0.25 μm; J&W Scientific, Folsom, CA, USA). The column oven temperature was held at 50 °C for 1 min, raised to 120 °C at 3 °C·min−1, and then increased to 240 °C at 10 °C·min−1 for 10 min. Helium (1.0 ml·min−1) was used as the carrier gas. Splitless injection (2 μl) was used with an injector temperature of 250 °C. The transfer line was set at 280 °C. Compounds were identified based on comparison with the retention times and mass spectra of synthetic standards. Windows NT/MASS Spectral Search Program (Version 1.7) software was used for the data analysis44,45.
GC-EAD
Headspace extracts (2 µl) were analysed using the Gas Chromatography-Electroantennographic Detection (GC-EAD) system: an Agilent Technologies 7890 N GC coupled with an electroantennogram detector (Syntech, Hilversum, The Netherlands). Column and oven temperature programs were as previously described for GC-MS. Injector and detector temperatures were 250 °C and 230 °C, respectively. Nitrogen was used as the carrier gas at a constant flow of 1.0 ml·min−1. The outlet of the GC column was split in a 1:2 ratio between the flame ionization detector (FID) and a cut mated H. vitessoides female antenna through a heated (280 °C) transfer line. The antenna was mounted in a holder with two metal electrodes using conductive gel (Spectra 360; Parker Laboratories, Fairfield, NJ, USA). The electrode was connected to a high impedance DC amplifier (IDAC-4; Syntech). Compounds eluting from the GC column were delivered to the mounted antenna through a glass tube (12 × 0.8 cm), carried by a humidified and purified supplemental airflow. The antennal signal and the FID signal were simultaneously recorded and analysed using Syntech software (Hilversum, The Netherlands). Each antenna used for the tests was cut from a different mated female and used only once. Each sample was tested three times.
Chemicals
Nonanal (97%), decanal (97%), and octanal (98%) were obtained from Fluka Production (Buchs, Switzerland). (Z)-3-hexenyl acetate (97%) and (Z)-3-hexen-1-ol (98%) were bought from Carl Roth (Karlsruhe, Germany). Hexyl acetate (99%), methyl benzoate (99%), 2-decen-1-ol (97%), and 1-octen-3-ol (98%) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). (Z)-β-ocimene (95%) was obtained from BOC Sciences (New York, USA). Compounds for which no standards were available were tentatively identified using the NIST-database42.
Wind tunnel bioassays
Assessments of the attractiveness of synthetic chemical blends to mated H. vitessoides females were carried out in a plexiglas wind tunnel (flight section: 200 × 60 × 60 cm). Incoming air was filtered through activated charcoal and was blown by a horizontal fan at 0.3 m·s−1 at the point of release of the moths. The upwind and downwind ends of the tunnel were covered with gauze to prevent escape of moths41,46. All bioassays were performed from 20:00 to 04:00, which is the oviposition peak period of H. vitessoides12. During the tests, temperature and relative humidity of the wind tunnel were kept at 25 ± 2 °C and 75 ± 5%, respectively.
On the basis of the results of the GC-EAD analyses, VOCs from the six different treatments of A. sinensis eliciting antennal responses in the female of H. vitessoides were formulated in blends for the wind tunnel tests. Six blends of synthetic compounds were prepared in the ratios of GC-EAD-active VOCs as emitted by the corresponding treatments (Table 2). Chemicals were diluted with redistilled hexane (Sigma-Aldrich, St. Louis, MO, USA). For each blend, the formulations contained 0.5 mg of the most abundant compound and the others compounds were added in the same proportion as in the natural volatile mixture. Preliminary tests confirmed that these concentrations were adequate to elicit moth responses in the wind tunnel. The blends were released into the wind tunnel by means of a green rubber septum. A septum treated with hexane only and no scent lure served as a control12,43,47.
Before the bioassays, the synthetic lures were loaded in individual rubber septa respectively. Each septum loaded with one of the test samples was placed in the centre of the upwind end of the tunnel (30 cm from upwind end), affixed to a holder, and used only once per day. In order to reduce the experimental error between different blends or same blends with different replications, all odour blends were deployed based on the same criteria. After each treatment, the flight section of the wind tunnel was washed with hexane, and then dried with an electric hair drier (HP 8200; Philips, Zhuhai, China)43,48.
One hour before the trial, all mated females were transferred to the wind tunnel room and allowed to acclimate to the conditions. Test females were introduced into the downwind end of the wind tunnel one by one. Three groups were run for each blend tested. The number of females tested in each group ranged from 25–30 depending on availability of mated females. Females were placed in a cylindrical gauze cage (diameter: 10 cm, height: 15 cm). The cylinder was closed with a solid lid on one side and placed in a holder at a height of 30 cm in the centre of the downwind end of the wind tunnel. At the beginning of the bioassays, the lid was removed, allowing the moths to leave the cage. Moth behaviour was scored as follows: (1) for >120 cm upwind oriented flight in the centre of the wind tunnel and (2) for coming within 5 cm of the odour source. The behaviour of each batch was observed for 20 min. Each female was used only once.
Y-tube olfactometry
We tested the attraction of predatory C. concinna to damaged and undamaged plant tissue in a glass Y-tube olfactometer. Undamaged A. sinensis plants served as a control, and damaged plants were the same damage treatments described in ‘Plant materials and treatments’. The olfactometer consisted of two glass chambers (diameter: 10 cm) that were each connected with one of the two 20-cm-long arms of the olfactometer, and joined with a 20-cm-long common arm. The odour sources (potted plant tissue) were placed inside clean roasting bags, which were then connected to the extremities of each arm: one arm served as a control (undamaged plants) and the other held the test material (H. vitessoides damaged plants). Fine-meshed nylon gauze was inserted at the ends of the two arms of the Y-tube to prevent insects from reaching the plant tissue. Moistened, activated-charcoal-filtered air with the odour source was pumped into each arm at a flow rate of 300 ml·min−1. All bioassays were conducted during the photophase, which is the feeding period of C. concinna. Temperature and relative humidity in the Y-tube olfactometer were maintained at 25 ± 2 °C and 75 ± 5%, respectively.
One hour before the start of the bioassays, groups of insects were transferred to the Y-tube room and allowed to acclimatize in the observation room inside glass vials (15 × 3 cm). For the observations, insects were placed individually at the beginning of the common arm and observed for 10 min. Behaviour was recorded as choosing the test odour or the control if the insects entered the respective chamber. If the insects remained in the common arm of the Y-tube it was recorded that no choice had been made49. For each bioassay, 30 replicates were performed. Each insect was used only once in the bioassays. After each test, the Y-tube was washed with distilled water, acetone, and alcohol (v/v 90%), and then dried with an electric hair drier (HP 8200; Philips, Zhuhai, China).
Data Analysis
Mean volatile concentrations in the headspace samples from different treatments, mean numbers of H. vitessoides females responding to each VOC blend in the wind tunnel, and percentage of C. concinna adults making each choice in Y-tube were each compared using one-way analysis of variance (ANOVA). Significant differences in the means were assessed using Tukey’s multiple range test (α = 0.01). Chi-square test was applied to analyse results from the Y-tube behavioural tests. To reduce the complexity of the multivariate VOC data, principal component analysis (PCA) was performed. PCA was applied to yield a 2D display of the multivariable data set and to graphically determine whether clustering of the six damage treatments (undamaged plants, mechanically damaged plants, and H. vitessoides damaged plants) occurred based on their overall VOC profiles50. All statistical analyses were performed using SPSS, version 16.0 (SPSS Inc. Chicago, IL, USA).
References
Dicke, M. Are herbivore-induced plant volatiles reliable indicators of herbivore identity to foraging carnivorous arthropods? Entomol. Exp. Appl. 91, 131–142 (1999).
Angioy, A. M., Desogus, A., Barbarossa, I. T., Anderson, P. & Hansson, B. S. Extreme sensitivity in an olfactory system. Chem. Senses 28, 279–284 (2003).
Anton, S., Dufour, M. C. & Gadenne, C. Plasticity of olfactory-guided behaviour and its neurobiological basis: lessons from moths and locusts. Entomol. Exp. Appl. 123, 1–11 (2007).
Bruce, T. J. A. & Pickett, J. A. Perception of plant volatile blends by herbivorous insects – Finding the right mix. Phytochemistry 72, 1605–1611 (2011).
Pare, P. W. & Tumlinson, J. H. Plant volatiles as a defense against insect herbivores. Plant Physiol. 121, 325–332 (1999).
Peñaflor, M. F. G. V. & Bento, J. M. S. Herbivore-induced plant volatiles to enhance biological control in agriculture. Neotrop. Entomol. 42, 331–343 (2013).
Zhang, S. F., Wei, J. N., Zhang, Z. & Kang, L. Rhythms of volatiles release from healthy and insect-damaged Phaseolus vulgaris. Plant Signaling & Behavior 8, e25759 (2013).
Dicke, M. & Loon, J. J. A. V. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 97, 237–249 (2000).
Sun, X. L., Gao, Y. & Chen, Z. M. Behavior regulation of herbivores by herbivore induced plant volaties (HIPVs). J. Appl. Entomol. 6, 1413–1422 (2012).
Poelman, E. H. et al. Hyperparasitoids use herbivore-induced plant volatiles to locate their parasitoid host. PLoS Biol. 10, e1001435 (2012).
National Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China. Part 1 185 (China Medical Science and Technology Press, 2015).
Qiao, H. L. et al. Antennal and behavioural responses of Heortia vitessoides females to host plant volatiles of Aquilaria sinensis. Entomol. Exp. Appl. 143, 269–279 (2012).
Su, Y. P. Biological characters of Heortia vitessoides. Journal of Chinese Medicinal Materials 17, 7–9 (1994).
Kalita, J., Bhattacharyya, P. R. & Nath, S. C. Heortia vitessoides Moore (Lepidoptera: Pyralidae) - A serious pest of Agarwood plant (Aquilaria malaccensis Lamk.). Geobios 29, 13–16 (2001).
Qiao, H. L. et al. Biological characteristics and occurrence patterns of Heortia vitessoides. Chinese Journal of Applied Entomology 50, 1244–1252 (2013).
De Moraes, C. M., Mescher, M. C. & Tumlinson, J. H. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410, 577–580 (2001).
Signoretti, A. G. C., Penaflor, M. F. G. V. & Bento, J. M. S. Fall armyworm, Spodoptera frugiperda (Smith, J. E.) (Lepidoptera: Noctuidae), female moths respond to herbivore-induced corn volatiles. Neotrop. Entomol. 41, 22–26 (2012).
Sarmento, R. A. et al. A herbivore that manipulates plant defence. Ecol. Lett. 14, 229–236 (2011).
Kappers, I. F., Hoogerbrugge, H., Bouwmeester, H. J. & Dicke, M. Variation in herbivory-induced volatiles among cucumber (Cucumis sativus L.) varieties has consequences for the attraction of carnivorous natural enemies. J. Chem. Ecol. 37, 150–160 (2011).
Zakir, A. et al. Specific response to herbivore-induced de novo synthesized plant volatiles provides reliable information for host plant selection in a moth. J. Exp. Biol. 216, 3257–3263 (2013).
Loughrin, J. H., Manukian, A., Heath, R. R., Turlings, T. C. & Tumlinson, J. H. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plant. Proc. Natl. Acad. Sci. USA 91, 11836–11840 (1994).
Röse, U. S. R. & Tumlinson, J. H. Volatiles released from cotton plants in response to Helicoverpa zea feeding damage on cotton flower buds. Planta 218, 824–832 (2004).
Price, P. W. et al. Interactions among three trophic levels: Influence of plants on interactions between insect herbivores and natural enemies. Annual Review of Ecology & Systematics 11, 41–65 (1980).
Wee, S. L., El-Sayed, A. M., Gibb, A. R., Mitchell, V. & Suckling, D. M. Behavioural and electrophysiological responses of Pantomorus cervinus (Boheman) (Coleoptera: Curculionidae) to host plant volatiles. Aust. J. Entomol. 47, 24–31 (2008).
Hegde, M. et al. Identification of semiochemicals released by cotton, Gossypium hirsutum, upon infestation by the cotton aphid, Aphis gossypii. J. Chem. Ecol. 37, 741–750 (2011).
Bezemer, T. M., Wagenaar, R., Dam, N. M. V., Putten, W. H. V. D. & Wäckers, F. L. Above- and below-ground terpenoid aldehyde induction in cotton, Gossypium herbaceum, following root and leaf injury. J. Chem. Ecol. 30, 53–67 (2004).
Kessler, A. & Baldwin, I. T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 291, 2141–2144 (2001).
Michereff, M. F. F. et al. Volatiles mediating a plant-herbivore-natural enemy interaction in resistant and susceptible soybean cultivars. J. Chem. Ecol. 37, 273–285 (2011).
Dicke, M. & Baldwin, I. T. The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci. 15, 167–175 (2010).
Mccormick, A. C., Unsicker, S. B. & Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 17, 303–310 (2012).
Bruce, T. J. A. Interplay between insects and plants: dynamic and complex interactions that have coevolved over millions of years but act in milliseconds. J. Exp. Bot. 66, 455–465 (2015).
Dudareva, N., Negre, F., Nagegowda, D. A. & Orlova, I. Plant Volatiles: recent advances and future perspectives. Crit. Rev. Plant Sci. 25, 417–440 (2006).
Turlings, T. C. J., Lengwiler, U. B., Bernasconi, M. L. & Wechsler, D. Timing of induced volatile emissions in maize seedlings. Planta 207, 146–152 (1998).
Wei, J. N., Zhu, J. & Le, K. Volatiles released from bean plants in response to agromyzid flies. Planta 224, 279–287 (2006).
Röse, U. S. R. & Tumlinson, J. H. Systemic induction of volatile release in cotton: how specific is the signal to herbivory? Planta 222, 327–335 (2005).
Hatano, E. et al. A herbivore-induced plant volatile interferes with host plant and mate location in moths through suppression of olfactory signalling pathways. BMC Biol. 13, 75 (2015).
Sun, X. L. et al. Volatiles Emitted from Tea Plants Infested by Ectropis obliqua Larvae Are Attractive to Conspecific Moths. J. Chem. Ecol. 40, 1080–1089 (2014).
Davis, T. S., Crippen, T. L., Hofstetter, R. & Tomberlin, J. K. Microbial Volatile Emissions as Insect Semiochemicals. J. Chem. Ecol. 39, 840–859 (2013).
Iii, L. W., Blackmer, J. L., Rodriguez-Saona, C. & Zhu, S. Plant volatiles influence electrophysiological and behavioral responses of Lygus hesperus. J. Chem. Ecol. 36, 467–478 (2010).
Borrero-Echeverry, F. et al. Flight attraction of Spodoptera littoralis (Lepidoptera, Noctuidae) to cotton headspace and synthetic volatile blends. Frontiers in Ecology and Evolution 3, 1–6 (2015).
Dong, H. C. et al. Flight tunnel responses of female grape berry moth (Paralobesia viteana) to host plants. J. Chem. Ecol. 34, 622–627 (2008).
Tasin, M. et al. Antennal and behavioral responses of grapevine moth Lobesia botrana females to volatiles from grapevine. J. Chem. Ecol. 31, 77–87 (2005).
Anfora, G., Tasin, M., Cristofaro, A. D., Ioriatti, C. & Lucchi, A. Synthetic grape volatiles attract mated Lobesia botrana females in laboratory and field bioassays. J. Chem. Ecol. 35, 1054–1062 (2009).
Condursoa, C., Verzeraa, A., Romeoa, V., Ziinoa, M. & Conteb, F. Solid-phase microextraction and gas chromatography mass spectrometry analysis of dairy product volatiles for the determination of shelf-life. Int. Dairy J. 18, 819–825 (2008).
Tasin, M. et al. Attraction of female grapevine moth to common and specific olfactory cues from 2 host plants. Chem. Senses 35, 57–64 (2010).
Dong, H. C. et al. Identification and field evaluation of grape shoot volatiles attractive to female grape berry moth (Paralobesia viteana). J. Chem. Ecol. 34, 1180–1189 (2008).
Lu, P. F., Huang, L. Q. & Wang, C. Z. Identification and field evaluation of pear fruit volatiles attractive to the oriental fruit moth, Cydia molesta. J. Chem. Ecol. 38, 1003–1016 (2012).
Najar-Rodriguez, A. J., Galizia, C. G., Stierle, J. & Dorn, S. Behavioral and neurophysiological responses of an insect to changing ratios of constituents in host plant-derived volatile mixtures. J. Exp. Biol. 213, 3388–3397 (2010).
Hern, A. & Dorn, S. A female-specific attractant for the codling moth, Cydia pomonella, from apple fruit volatiles. Naturwissenschaften 91, 77–80 (2004).
Najar-Rodriguez, A., Orschel, B. & Dorn, S. Season-long volatile emissions from peach and pear trees in situ, overlapping profiles, and olfactory attraction of an oligophagous fruit moth in the laboratory. J. Chem. Ecol. 39, 418–429 (2013).
Acknowledgements
We thank Mr. Rui Wang from the Institute of Zoology, Chinese Academy of Sciences, who provided technical assistance and advice for GC-EAD. Mr. Xiang-ming Li and Ms. Rong-Min Qin from Huazhou Green Life Co. Ltd provided help with experimental materials. We would like to thank Editage (www.editage.com) for reviewing the manuscript to improve the English. We also would like to thank Dr. Uray, PhD, ELS, who is a native English speaker and an experienced professional scientific manuscript editor for SCINET Co., Ltd. for revising and reviewing the manuscript to improve clarity and readability. This work was supported by the National Natural Science Foundation of China (grant no. 81774015, 31570643, Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2016-I2M-3-017).
Author information
Authors and Affiliations
Contributions
H.Q. designed and performed the experiments, analysed the data, and wrote the paper. P.L. performed the experiments. S.L., C.X., K.G., R.X. provided scientific guidance. J.C. designed the experiments and provided scientific support and guidance.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qiao, H., Lu, P., Liu, S. et al. Volatiles from Aquilaria sinensis damaged by Heortia vitessoides larvae deter the conspecific gravid adults and attract its predator Cantheconidea concinna. Sci Rep 8, 15067 (2018). https://doi.org/10.1038/s41598-018-33404-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-33404-z
- Springer Nature Limited