Abstract
Last year we started this series of end of year summaries of papers published in the 2014 issues of the Journal Of Clinical Monitoring And Computing with a review on near infrared spectroscopy (Scheeren et al. in J Clin Monit Comput 29(2):217–220, 2015). This year we will broaden the scope and include papers published in the field of tissue oxygenation and microcirculation, or a combination of both entities. We present some promising new technologies that might enable a deeper insight into the (patho)physiology of certain diseases such as sepsis, but also in healthy volunteers. These may help researchers and clinicians to evaluate both tissue oxygenation and microcirculation beyond macro-hemodynamic measurements usually available at the bedside.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Monitoring tissue oxygenation
Ensuring an adequate tissue oxygenation may be considered the “holy grail” of cardiovascular management, and all variables we routinely measure in the perioperative and critical care setting (stroke volume, heart rate, cardiac output, Hb, SaO2, paO2, DO2, etc.) contribute to this ultimate goal. Tissue oxygenation is the oxygen tension measured at the capillary level, the interstitial space and even intracellular in the mitochondria, and it gives information about the balance between local oxygen delivery (DO2) and oxygen consumption (VO2) [1]. While the oxygen tension within an individual organ remains reasonably comparable across species, there are intra-organ differences, depending on regional variations in organ flow and activity [2].
There are quite a number of methodologies available for estimating or measuring tissue oxygenation at the different levels, such as Clark type electrodes, near infrared spectroscopy (NIRS), reflectance spectroscopy, and mitochondrial pO2 measurements. The most popular of these is certainly NIRS [3], a fact that is reflected by the number of papers published last year in the JCMC.
In the August issue, Koch et al. [4] presented an interesting prospective observational study in which they looked at tissue oxygenation at two sites (forehead and thenar muscle) using NIRS technology in 50 patients with severe sepsis or septic shock. These regional oxygenation values (rSO2) were compared to central venous oxygen saturation (ScvO2), which often serves as a treatment goal for optimizing tissue oxygenation in these patients [5]. In particular, the authors wanted to know if central (ScvO2) and regional (rSO2) indicators of tissue oxygenation are correlated and if they could be used to predict outcome in septic patients. They found that mean baseline rSO2 in their septic patients was 60 ± 11 %, which was considerably lower than that observed in non-septic patients undergoing major surgery (86 ± 6 %) [6] or in non-septic ICU patients (80 ± 7 %) [7]. There was a moderate correlation between frontal and thenar rSO2, however, no relation to outcome (i.e. similar values between survivors and non-survivors with a hospital mortality of 52 %). Similarly, there was a weak correlation between thenar rSO2 and ScvO2, as also described previously [6]. Nevertheless, frontal rSO2 was able to predict a ScvO2 below 70 %. Surprisingly, ScvO2 was higher in non-survivors (76 ± 8 %) than in survivors (71 ± 8 %) at baseline, suggesting an oxygen extraction or utilization problem in the first group. As such, a high baseline ScvO2 (>70 %) but not rSO2 was predictive of mortality, particularly when combined with a lactate level >2.5 mmol/l. This interesting finding raises the question if targeting a ScvO2 > 70 % (as described in current guidelines such as the surviving sepsis campaign) [8] is really beneficial for the patient with sepsis. Unfortunately, the authors did not perform a vascular occlusion test (VOT) in their patients, which would have given further information on muscle metabolism (VO2, derived from the descending slope) and of (micro)vascular reperfusion (vasoreactivity, derived from the ascending slope) (see below). Both slopes were related to outcome, with non-survivors having increased descending slopes [9] and lower reperfusion slopes [7].
In the accompanying editorial comment to this paper, Mesquida points out that regional rSO2 and global ScvO2 are not reflecting exactly the same entity, so that the usefulness of predicting the one by the other may be questionable [10]. Yet, unlike ScvO2 measurements, rSO2 measurements are purely non-invasive and give an instantaneous result, which qualifies NIRS as a screening tool for detecting tissue hypoxia early in the resuscitation process. Instead of using rSO2 measurements to predict ScvO2 measurements, Mesquida suggests an integrative approach, combining global and regional markers of tissue oxygenation to detect tissue dysoxia at the bedside [10]. Furthermore, Mesquida evaluates the association between ScvO2 and lactate with mortality by describing four scenarios: (1) adequate global DO2 and cellular metabolism (normal lactate and normal ScvO2); (2) oxygen deficit (normal lactate and low ScvO2); (3) oxygen debt (high lactate and low ScvO2); and (4) impaired oxygen extraction (high lactate and high ScvO2). The latter scenario was associated with the highest mortality (80 %) in the Koch study [4], although an inadequate DO2 was probably not the underlying problem. This important finding raises doubts on the benefit of the current clinical practice of maximizing DO2 during the resuscitation of septic patients.
In the August issue, Murphy and colleagues evaluated the utility of cerebral oxygenation (ScO2) measurements obtained by NIRS in a small group of patients undergoing hepato-biliary surgery [11]. In these patients, increased bilirubin concentrations can interfere with the NIRS measurements by absorbing infrared light at similar wavelengths as deoxygenated hemoglobin, making the obtained ScO2 values unreliably low. In view of the well-known fact that changes in ScO2 values from individual baseline are more important than absolute ScO2 values, the authors investigated if the time course of ScO2 values is affected in patients with high bilirubin levels, other than starting from a lower baseline value. Patients were divided into two groups using a ScO2 cut-off value of 51 %, which has been identified as threshold value for detecting cerebral ischemia [12]. They found that although starting from different baseline levels, the reactions to preoxygenation as well as the intraoperative time course of ScO2 values were similar between groups. Therefore, the authors conclude that trends in cerebral oximetry may also be useful in patients with liver dysfunction and increased bilirubin levels, even if absolute ScO2 values are falsely low and thus do not per se indicate cerebral ischemia.
In the same issue, Ritzenthaler et al. [13] report on a small case series of ScO2 variation in three patients undergoing mechanical thrombectomy after acute ischemic stroke. As expected, baseline ScO2 was lower in the infarcted compared to the non-infarcted side, resulting in an interhemispheric difference >5 %. After successful recanalization (which occurred in two of the three patients), ScO2 at the infarcted side increased by about 10 %, resulting in a decrease of the interhemispheric difference. In contrast, in the patient where the thrombectomy failed, ScO2 values remained constant. The authors conclude that cerebral NIRS monitoring could be used to monitor the success of recanalization therapy of acute ischemic stroke, and that the continuous ScO2 readings might provide additional information to instantaneous CT or MRI imaging. It has to be noted, however, that ScO2 measurements as usually performed at the forehead typically assess a sample volume of the watershed area between the anterior and middle cerebral artery territory, and may not detect ischemic events in other regions of the brain, particularly those in the posterior region.
A more traditional approach is the monitoring of ScO2 during carotid endarterectomy (CEA). In a prospective observational study involving 69 patients undergoing CEA, Perez et al. [14] followed changes in ScO2 as well as processed EEG responses to carotid clamping and shunting. In general, shunting of the clamped carotid artery is performed in about a quart of all CEA cases, and, in view of the potential complications associated with the shunting procedure, there is not enough evidence to support or refute its use as of yet. Detection of cerebral ischemia by monitoring ScO2 or processed EEG might help identify patients who benefit from carotid shunting when this procedure is performed under general anesthesia. The authors measured bilateral BIS (bispectral index) and ScO2 and found that after clamping ScO2 at the surgical-side was significantly lower than at the contralateral side until the shunt was removed and blood flow was restored in the repaired carotid artery. BIS decreased bilaterally to a value significantly below baseline following clamping but returned to a value near baseline following shunt activation with no differences between sides. In other words: while ScO2 measurements could clearly differentiate between surgical and contralateral side, BIS (although measured bilaterally) could not. Furthermore, the minimum ScO2 and BIS values observed after carotid clamping did not correlate with the degree of carotid stenosis determined preoperatively. A nice side-effect discussed by the authors relates to the discussion on “extracranial contamination” of the NIRS signal; [15] during CEA, the common, internal and external carotid arteries are clamped prior to insertion of the shunt. After the shunt is placed, the common and internal carotid arteries are unclamped, while the external carotid artery remains clamped, resulting in decreased perfusion of superficial and extracranial tissues. The study results clearly show that ScO2 increases after activation of the shunt due to actual changes in intracranial blood flow. Important limitations of the study include the lack of blood pressure control and, due to the pure observational character, the fact that no conclusions can be drawn on the possible impact of either monitoring on neurological outcome. Finally, a cost-benefit analysis should be performed to evaluate if a reduction in neurological complications justifies the additional costs of the monitoring.
In the same issue, Eichhorn and colleagues report on the ScO2 response during hypoxia in apnea divers [16]. What at first view looks like a pure physiological study with little clinical applicability, is actually of high relevance to the clinical setting of anesthesia: the authors intended to mimic dynamic hypoxic situations such as those occurring during difficult intubation, “cannot ventilate cannot intubate” situations or laryngospasm, as well as during cardiopulmonary resuscitation (CPR). For this purpose, they asked 10 experienced apneic divers to perform a maximal breath hold manoeuvre. They found that during apnea both peripheral oxygen saturation (SpO2) and ScO2 significantly decreased with a close correlation of both variables after normalization to individual baseline values. More importantly, ScO2 significantly faster returned to baseline values after the end of apnea than SpO2, suggesting that cerebral oximetry might be useful under conditions of airway problems and in pulseless CPR situations where SpO2 monitoring fails. In a second set of experiments, the authors looked also at peripheral tissue oxygenation as measured via NIRS above the quadriceps muscle, and found that these measurements decreased even earlier than SpO2 and ScO2 values during apnea, probably as a result of peripheral vasoconstriction. This theory is supported by the finding that the hemoglobin volume (measured also with NIRS) decreased in the peripheral muscle, whereas it remained constant in the brain, probably due to cerebral blood flow autoregulation.
In an animal study, Kurita et al. [17] investigated the effects of beta-blockade and hemodilution on ScO2. It is known that during acute hemodilution beta-blockers reduce ScO2, probably by blunting the cardiac response to hemodilution. This has clinical implications, for instance in situations where controlled hypotension is induced with beta-blockers and surgical bleeding occurs. Using anesthetized pigs allowed the authors to systematically combine different beta-blocker dosages and hemodilution conditions, and in addition looking at the impact of cardio-selectivity on ScO2 by using two different short-acting beta-blockers, one more cardio-selective (landiolol) than the other (esmolol). While both beta-blockers had no effect on ScO2 before hemodilution, they decreased ScO2 dose-dependently at the different stages of hemodilution with no difference between drugs. While the effects of the cardio-selective landiolol on ScO2 were reversible after stopping drug infusion, ScO2 remained below preinfusion values after stopping esmolol during hemodilution. Of note, hemodilution up to 66 % per se did not reduce ScO2 in this animal model, since cardiac output compensatorily increased by about 50 % to maintain DO2. When these experimental findings were transferred to the clinical setting, they imply that perioperative ScO2 monitoring might be particularly useful in patients receiving beta-blockers and undergoing surgery where major bleeding might occur. Furthermore, it should be investigated further if cardio-selective beta-blockers impair cerebral oxygenation to a lesser extent under these conditions than unselective beta-blockers.
2 Combined monitoring of microcirculation and tissue oxygenation
A hot topic in perioperative medicine and intensive care since several years is the monitoring of fluid responsiveness. Klijn et al. [18] tried to evaluate how monitoring of the microcirculation (tissue perfusion) and tissue oxygenation can be used for this purpose. In a cohort of 35 critically ill patients with sepsis, they measured sublingual microcirculatory perfusion with side-stream dark field (SDF) imaging and also skin perfusion and oxygenation by combined laser Doppler and reflectance spectroscopy, a diagnostic tool for assessing microvascular blood flow and tissue oxygen consumption at the bedside [19]. By defining fluid responsiveness as an increase in stroke volume (SV) by >5 % in response to a 250 ml colloid infusion, the authors found that in fluid responders, all markers of microcirculatory perfusion and tissue oxygenation increased, despite the fact that initial fluid resuscitation had been performed before starting the current fluid bolus. They conclude that their non-invasive methods to assess the microcirculation and tissue oxygenation were not inferior to the traditional, invasive hemodynamic measurements usually performed to monitor fluid responsiveness in clinical practice, and could therefore help guiding fluid therapy particularly in situations where the risk of harmful fluid overloading is increased (such as in sepsis). The fact that the changes in microcirculatory flow and tissue oxygenation were relatively low (albeit significant) might be explained by the facts that 1) the fluid challenge was performed after initial volume resuscitation and 2) that the sublingual microcirculation was already “hyperdynamic” before the fluid challenge, a finding typical for the later stage of sepsis [20]. Finally, the techniques used by the authors do not belong to the current clinical armamentarium and are rather research tools as of yet, mainly due to the necessity for off-line analysis and substantial intra- and interrater variability. Future developments may help bringing these techniques to the bedside, allowing for routine measurements and, most importantly, for collecting data showing an impact on patient outcome.
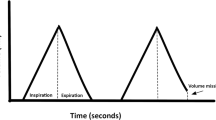
As mentioned above, the vascular occlusion test in combination with NIRS monitoring at the upper or lower extremity can reveal valuable information on the reaction of the microcirculation. After occlusion of the vessels supplying blood flow to the extremity where tissue oxygenation is measured by inflating a cuff above systolic blood pressure, the descending or desaturation slope gives information about the oxygen metabolism (VO2) of the muscle under the NIRS probe. After deflation of the cuff following limb ischemia, the upstroke or reperfusion slope of the tissue oxygenation curve can be taken as a measure of microvascular reactivity and recruitment, i.e. the ability to washout the stagnant deoxygenated blood from the measurement volume and replace it by “fresh”, oxygenated blood [21–23].
Two studies published in the JCMC last year used the VOT in combination with peripheral NIRS measurements [23, 24]. In the first study, Zamparini et al. [24] examined if the VOT could be used to differentiate NIRS-derived vascular reactivity between smokers and non-smokers. For this purpose, they included 20 healthy volunteers of both sexes (10 smokers and 10 non-smokers) and hypothesized that endothelial dysfunction usually seen in smokers could be detected by revealing microcirculatory dysfunction using NIRS and VOT above a leg muscle. However, there were absolutely no differences between smokers and non-smokers, neither in desaturation slope during ischemia nor in resaturation rates during reperfusion. Possible explanations provided by the authors include the inability or lack of sensitivity of NIRS measurements to detect smoking-induced endothelial dysfunction on the one hand and a “contamination” of the NIRS-derived hemoglobin oxygen saturation measurements by muscular myoglobin on the other. Furthermore, the choice of otherwise healthy, young volunteers (mean age 20 years) with a relatively short smoking history (10 pack-years) might have contributed to the negative findings.
In the second study on NIRS VOT published in the April issue, Lee et al. investigated if the NIRS device used matters when a VOT is performed. As the VOT has initially been described using the InSpectra® device (Hutchinson Technology, USA) and thenar rSO2 measurements, the authored wondered if other NIRS devices (primarily designed for measuring ScO2) could be used interchangeably for this purpose. In 20 healthy volunteers they compared rSO2 measurements derived from two different devices (InSpectra® and INVOS®, Covidien) during a VOT in the same subject. While the baseline values were significantly lower with the INVOS® as compared to those of the InSpectra® (75 vs. 82 %), the minimum rSO2 during VOT was reached earlier with the INVOS®. Similarly, the desaturation and reoxygenation rates were higher as measured with the INVOS®. These results show that either device can be used for measuring rSO2 during a VOT, a finding that has been reported earlier [12, 25], but that device-specific differences in baseline values and dynamic changes should be considered.
3 Monitoring the microcirculation
The microcirculation is a central part of the cardiovascular system consisting of blood vessels with a diameter of <150 µm. The main function of the microcirculation is to deliver oxygen (DO2) to the cells and maintain tissue oxygenation. The normal microcirculation is characterized by a dense network of perfused capillaries with minimal heterogeneity, most of the capillaries being perfused even though flow in the various capillaries varies according to metabolic needs of the surrounding tissues. Adaptation to metabolic needs occurs by opening and closing capillaries, and adapting the velocity of circulating cells within these. Modulation of precapillary sphincters is partly under the influence of systemic factors, with sympathetic stimulation and circulating substances, whereas the fine-tuning perfusion is regulated by local factors that include direct stimulation of endothelial cells by backward communication and local release of nitric oxide by red blood cells under hypoxic conditions.
In recent years, a large body of knowledge supports a central pathophysiological importance of the microcirculation in the development of organ failure. Microcirculatory alterations are more severe in non-survivors than in survivors. Improvements in microvascular perfusion occurring in response to goal resuscitation therapies are associated with subsequent improvements in organ function while organ function further deteriorated when microvascular perfusion failed to improve or even worsened. Thus, the state of the microcirculation might be considered an important prognostic indicator.
For many years, the direct study of the microcirculation was restricted to animal experimentation. The recent introduction of video microscopy using hand-held microscopes allows the non-invasive visualization of the microcirculation in patients. The analysis of the microcirculation should include parameters of vessel density (total and perfused vascular densities), perfusion [proportion of perfused vessels (PPVs) and microvascular flow index (MFI)], and heterogeneity [26]. Particularly, the sublingual region has been frequently assessed for video microscopy due to its good accessibility and its close correlation with other vascular beds, e.g. the intestinal perfusion. Recently, a new device based on incident dark-field (IDF) imaging (Cytocam) was developed for this application, with improved optical lenses, a high-resolution computer controlled image sensor, and an application for automatic analysis [27]. In a study on preterm neonates, van Elteren and colleagues compared this new IDF imaging technique to the older SDF imaging method [28]. Neonates were chosen since their reduced skin thickness facilitates transcutaneous non-invasive measurements on the inner upper arm. The authors found that IDF imaging visualized 20 % more vessels (particularly small vessels) than the older SDF device, resulting in a higher observed vessel density and a lower perfusion (as determined by the proportion of perfused vessels, PPV). When the authors looked at the imaging quality, the IDF images scored better than the SDF video images (25 % more optimal scores using the standardized imaging quality score) [29]. This may be due to technical advancement in the image acquisition as well as in the lower weight of the handheld camera (120 vs. 350 g), allowing a reduction of motion and pressure artefacts.
In an accompanying editorial comment, Lehmann and colleagues point out that both cameras (i.e. IDF and SDF) have a different optical resolution and a distinctive size of observation area, so that these different microcirculatory windows should affect the comparison, even if the same off-line analysis software was used [30]. They also expect that the introduction of automated analysis software packages will initiate more validation studies as a prerequisite to the introduction of these promising technologies into clinical practice.
Another step forward is made by the group of Harms et al., who extended their microcirculatory approach by looking “even further down the road” into the cell, more precisely into mitochondrial function [31]. The authors used a technique that was developed earlier by this group, namely the so-called Protoporphyrin IX—Triplet State Lifetime Technique. This technique, the details of which cannot be comprehensively reproduced in this review, provides a new approach for measuring mitochondrial function and oxygen tension (mitoPO2) in vivo. In the current paper, the authors describe a modification of this invasive technique for transcutaneous application. Furthermore, they combined the technique with what they call oxygen disappearance rate (ODR) measurements, i.e. measuring the decline in mitoPO2 after pressure-induced cessation of blood supply (and thus DO2). From these ODR measurements they derive values of mitochondrial oxygen consumption (mitoVO2). In their paper, the authors present two sets of experiments, an animal study in rats (with or without lipopolysaccharide, LPS) and a volunteer study.
In the rats, the authors determined mitoPO2 and mitoVO2 in different organs (skin, liver and buccal mucosa) and compared this to skin perfusion and oxygenation as assessed by combined laser Doppler and reflectance spectroscopy [19]. While absolute values for skin oxygenation differed from those of the other organs, mitoVO2 (but not mitoPO2) decreased in all organs after LPS. Nevertheless, the authors conclude from this that the skin can be used as a substitute for other less accessible target organs to monitor alterations in mitochondrial respiration in critical illness like sepsis and septic shock. Although mitoPO2 represents the lowest end of the oxygen distribution in tissues [2], the values found by the authors were surprisingly high (about 50 mmHg for skin), but were confirmed with reflectance spectroscopy. In the volunteer part of the study, the skin measurements were repeated and revealed comparable results to those from rat skin, demonstrating the feasibility of the non-invasive technology to assess mitochondrial function for use in humans.
Taken together, with the help of new technologies as described above, researchers (and possibly in the near future also clinicians) do no longer have to rely on macro-hemodynamic measurements only when it comes to evaluating both the microcirculation and tissue oxygenation, pending that these technologies find their way and acceptance in clinical practice.
References
De Santis V, Singer M. Tissue oxygen tension monitoring of organ perfusion: rationale, methodologies, and literature review. Br J Anaesth. 2015;115(3):357–65. doi:10.1093/bja/aev162.
Scheeren TW, Schober P, Schwarte LA. Monitoring tissue oxygenation by near infrared spectroscopy (NIRS): background and current applications. J Clin Monit Comput. 2012;26(4):279–87. doi:10.1007/s10877-012-9348-y.
Dyson A, Singer M. Tissue oxygen tension monitoring: will it fill the void? Curr Opin Crit Care. 2011;17(3):281–9. doi:10.1097/MCC.0b013e328344f1dc.
Koch C, Röhrig R, Monz T, Hecker A, Uhle F, Schneck E, Weigand MA, Lichtenstern C. Prospective evaluation of regional oxygen saturation to estimate central venous saturation in sepsis. J Clin Monit Comput. 2015;29(4):443–53. doi:10.1007/s10877-015-9683-x.
van Beest P, Wietasch G, Scheeren T, Spronk P, Kuiper M. Clinical review: use of venous oxygen saturations as a goal: a yet unfinished puzzle. Crit Care. 2011;15(5):232. doi:10.1186/cc10351.
Spruit RJ, Schwarte LA, Hakenberg OW, Scheeren TW. Association of intraoperative tissue oxygenation with suspected risk factors for tissue hypoxia. J Clin Monit Comput. 2013;27(5):541–50. doi:10.1007/s10877-013-9460-7.
Creteur J, Carollo T, Soldati G, Buchele G, De Backer D, Vincent JL. The prognostic value of muscle StO2 in septic patients. Intensive Care Med. 2007;33(9):1549–56. doi:10.1007/s00134-007-0739-3.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi:10.1097/CCM.0b013e31827e83af.
Orbegozo Cortes D, Puflea F, Donadello K, Taccone FS, Gottin L, Creteur J, Vincent JL, De Backer D. Normobaric hyperoxia alters the microcirculation in healthy volunteers. Microvasc Res. 2015;98:23–8. doi:10.1016/j.mvr.2014.11.006.
Mesquida J. Evaluating tissue oxygenation at the bedside: Global, regional, or both? J Clin Monit Comput. 2015;29(4):431–3. doi:10.1007/s10877-015-9690-y.
Murphy N, Fröhlich S, Kong T, Boylan JF, Conlon N. Utility of near infrared light to determine tissue oxygenation during hepato-biliary surgery. J Clin Monit Comput. 2015;29(5):613–9. doi:10.1007/s10877-014-9642-y.
Scheeren TW, Bendjelid K. Journal of clinical monitoring and computing 2014 end of year summary: near infrared spectroscopy (NIRS). J Clin Monit Comput. 2015;29(2):217–20. doi:10.1007/s10877-015-9689-4.
Ritzenthaler T, Cho TH, Luis D, Berthezene Y, Nighoghossian N. Usefulness of near-infrared spectroscopy in thrombectomy monitoring. J Clin Monit Comput. 2015;29(5):585–9. doi:10.1007/s10877-014-9636-9.
Perez W, Dukatz C, El-Dalati S, Duncan J, Abdel-Rasoul M, Springer A, Go MR, Dzwonczyk R. Cerebral oxygenation and processed EEG response to clamping and shunting during carotid endarterectomy under general anesthesia. J Clin Monit Comput. 2015;29(6):713–20. doi:10.1007/s10877-014-9657-4.
Davie SN, Grocott HP. Impact of extracranial contamination on regional cerebral oxygen saturation: a comparison of three cerebral oximetry technologies. Anesthesiology. 2012;116(4):834–40. doi:10.1097/ALN.0b013e31824c00d7.
Eichhorn L, Erdfelder F, Kessler F, Doerner J, Thudium MO, Meyer R, Ellerkmann RK. Evaluation of near-infrared spectroscopy under apnea-dependent hypoxia in humans. J Clin Monit Comput. 2015;29(6):749–57. doi:10.1007/s10877-015-9662-2.
Kurita T, Morita K, Sato S. Evaluation of near infrared spectroscopy for detecting the beta blocker-induced decrease in cerebral oxygenation during hemodilution in a swine model. J Clin Monit Comput. 2015;29(6):779–88. doi:10.1007/s10877-015-9667-x.
Klijn E, van Velzen MH, Lima AP, Bakker J, van Bommel J, Groeneveld AB. Tissue perfusion and oxygenation to monitor fluid responsiveness in critically ill, septic patients after initial resuscitation: a prospective observational study. J Clin Monit Comput. 2015;29(6):707–12. doi:10.1007/s10877-014-9653-8.
Scheeren TW. Monitoring the microcirculation in the critically ill patient: reflectance spectroscopy. Intensive Care Med. 2011;37(6):1045–6. doi:10.1007/s00134-011-2197-1.
De Backer D, Donadello K, Sakr Y, Ospina-Tascon G, Salgado D, Scolletta S, Vincent JL. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med. 2013;41(3):791–9. doi:10.1097/CCM.0b013e3182742e8b.
Bernet C, Desebbe O, Bordon S, Lacroix C, Rosamel P, Farhat F, Lehot JJ, Cannesson M. The impact of induction of general anesthesia and a vascular occlusion test on tissue oxygen saturation derived parameters in high-risk surgical patients. J Clin Monit Comput. 2011;25(4):237–44. doi:10.1007/s10877-011-9301-5.
Futier E, Christophe S, Robin E, Petit A, Pereira B, Desbordes J, Bazin JE, Vallet B. Use of near-infrared spectroscopy during a vascular occlusion test to assess the microcirculatory response during fluid challenge. Crit Care. 2011;15(5):R214. doi:10.1186/cc10449.
Lee JH, Park YH, Kim HS, Kim JT. Comparison of two devices using near-infrared spectroscopy for the measurement of tissue oxygenation during a vascular occlusion test in healthy volunteers (INVOS(R) vs. InSpectra). J Clin Monit Comput. 2015;29(2):271–8. doi:10.1007/s10877-014-9595-1.
Zamparini G, Butin G, Fischer MO, Gerard JL, Hanouz JL, Fellahi JL. Noninvasive assessment of peripheral microcirculation by near-infrared spectroscopy: a comparative study in healthy smoking and nonsmoking volunteers. J Clin Monit Comput. 2015;29(5):555–9. doi:10.1007/s10877-014-9631-1.
Nygren A, Rennerfelt K, Zhang Q. Detection of changes in muscle oxygen saturation in the human leg: a comparison of two near-infrared spectroscopy devices. J Clin Monit Comput. 2014;28(1):57–62. doi:10.1007/s10877-013-9494-x.
De Backer D, Hollenberg S, Boerma C, Goedhart P, Buchele G, Ospina-Tascon G, Dobbe I, Ince C. How to evaluate the microcirculation: report of a round table conference. Crit Care. 2007;11(5):R101.
Aykut G, Veenstra G, Scorcella C, Ince C, Boerma C (2015) Cytocam-IDF (incident dark field illumination) imaging for bedside monitoring of the microcirculation. Intensive Care Med. 2015;3(1):4. doi:10.1186/s40635-015-0040-7
van Elteren HA, Ince C, Tibboel D, Reiss IK, de Jonge RC. Cutaneous microcirculation in preterm neonates: comparison between sidestream dark field (SDF) and incident dark field (IDF) imaging. J Clin Monit Comput. 2015;29(5):543–8. doi:10.1007/s10877-015-9708-5.
Massey MJ, Shapiro NI. A guide to human in vivo microcirculatory flow image analysis. Crit Care. 2016;20(1):35. doi:10.1186/s13054-016-1213-9.
Lehmann C, Sardinha J, Mukhtar AM. Sidestream versus incident dark field imaging: how to compare two different technologies to study the microcirculation? J Clin Monit Comput. 2015;29(5):539–40. doi:10.1007/s10877-015-9740-5.
Harms FA, Bodmer SI, Raat NJ, Mik EG. Cutaneous mitochondrial respirometry: non-invasive monitoring of mitochondrial function. J Clin Monit Comput. 2015;29(4):509–19. doi:10.1007/s10877-014-9628-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Scheeren, T.W.L. Journal of Clinical Monitoring and Computing 2015 end of year summary: tissue oxygenation and microcirculation. J Clin Monit Comput 30, 141–146 (2016). https://doi.org/10.1007/s10877-016-9846-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-016-9846-4