Abstract
In the present study we reported, for the first time, the gamma irradiation induced synthesis of chitosan/Au/bioactive glass (CS/Au/BG) nanocomposite. The bioactive glass (BG), with the composition 45% SiO2, 32.5% CaO, 15% Na2O, and 7.5% P2O5 wt% was synthesized through the sol–gel technique. XRD, SEM, EDX, and elemental mapping images were utilized to evaluate the structure of pure BG and CS/Au/BG nanocomposite. The antimicrobial efficacy was evaluated by zone of inhibition (ZOI), minimum inhibitory concentration (MIC), growth curve assay, and Ultraviolet irradiation effect. Investigation was carried on the antibiofilm effectiveness. Membrane leakage as well as SEM imaging were used to evaluate the antibacterial reaction mechanism. The crystallite size of CS/Au/BG nanocomposite was determined via Scherer equation as 22.83 nm. CS/Au/BG possessed the most ZOI activity against the tested microbes. The highest inhibition % of BG, and CS/Au/BG nanocomposite was investigated for S. aureus (15.65%, and 77.24%), followed by C. albicans (13.32%, and 64.75%). The quantity of protein leakage was directly-proportional after increasing the concentration of BG, and CS/Au/BG and counted to be 70.58, and 198.25 µg/mL, respectively (after applied 10 mg/mL). The promising results suggested the use of novel CS/Au/BG nanocomposite as an encourage candidate for wastewater treatment application against pathogenic microbes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biocomposites are an essential type of materials in tissue engineering because they activate host defence mechanisms in the biological milieu [1]. The primary goal of these materials is to rebuild any damaged tissue or organ [2]. Bioactive glass (BG) is an excellent candidate product for use as a bioactive, biodegradable, and antibacterial component in biocomposite compounds. BG is favorable fabrics for smooth and a hard tissue engineering and repair [3, 4], antimicrobial, and antibiofilm agents [5, 6], drug delivery and ionic therapy [7], dentistry and wound healing [8, 9], bone-filling materials in orthopaedic and dental surgery [10], agriculture [11], for plant protection [12], and in food industry as a coating for seafood and meat products [13]. Due to bio-potentiality and distinct surface area; naturally connected for the development of positively porous and promising BG in nanoscale form [14]. Furthermore, when used in the framework of a restorative composite, it has the ability to form apatite layers in bodily fluid and saliva, which might prevent further tooth decay [15, 16].
Infectious and pathogenic microbes have the ability to form biofilm polymer as exopolysaccharides which serve as a shielding hard material and protect the pathogenic microbes from the hard conditions such as disinfectants, antibiotics, and so on [17]. The BG possessed the ability to act as antibiofilm agents [18, 19], which penetrate the biofilm produced by the pathogenic microbes and interact with the polysaccharides secreted by the microbes and interfere its production as a promising way for increasing the inhibition % of the biofilm production [20].
Chitosan (CS) is an effective ingredient that is utilized in the construction of biocomposites with bioactive glass. Bioactive glass and chitosan composites have been documented for a various applications, including orthopaedic implants, periodontal regeneration products, membranes, tissue regeneration, and medical chemicals. Chitosan’s notable characteristics, which including non-toxicity, high absorption, biodegradability, compatibility, and cheap cost, had making it an effective and cost-effective dental and orthopaedic component [21, 22].
The use of metal nanoparticles in particular has been thoroughly examined in recent literature, yielding a diverse range of fabrication techniques, including physical processes [23], wet chemistry [24], and then so green chemistry strategies [25], yielding monometallic, bimetallic, or even trimetallic structures [26]. They have a wide range of uses due to their exceptional features, including catalysis [27], sensors [28], and cancer therapy [29]. Furthermore, their antibacterial capabilities are widely used in industries such as food [30], textiles [25], and biomedicine [31]. Au NPs are highly conductive metal nanoparticles that are employed in a variety of applications including electronics, piezoelectricity, optoelectronic devices, capacitors, biomedicine, biosensors, and photocatalysis [32,33,34,35,36,37].
Since hydrated electrons released by water radiolysis are efficient reduction species, the radiation approach has shown to be an accurate approach for producing metal nanoparticles with size control. The basic benefit of radiolysis is that it performs under extremely basic physico-chemical conditions (room Temp., no chemical reducing agents, diluted aqueous solutions, sterile production). Furthermore, metal nanoparticles formed only by reducing species are uniformly distributed in the solution [38, 39].
Overall, in the present study we reported, for the first time, the gamma irradiation induced synthesis of CS/Au/BG nanocomposite. The nano-bioactive glass (BG), with the composition (45% SiO2, 32.5% CaO, 15% Na2O, and 7.5% P2O5 wt%) was synthesized through the sol–gel technique. The structure of pure BG and CS/Au/BG nanocomposite were identified and validated through important techniques (XRD, SEM, EDX, and elemental mapping images). Finally, the fabricated samples were investigated for their antimicrobial potential after conducting the ZOI, MIC, UV exposure effect, and kinetic study through growth curve. On the other hand, the potential of samples to be a good antibiofilm agents had been estimated via tube method assay, and the estimated antibacterial mode of action was conducted through the assay of membrane leakage and confirmed by the imaging through the SEM technique.
Materials and Methods
Materials
Chitosan (CS) was purchased from Sigma Aldrich. Acetic acid (96%), tetraethyl orthosilicate 98% (TEOS), sodium phosphate (Na2HPO4, 98%), calcium hydroxide (Ca(OH)2, 98%), and Gold chloride trihydrate HAuCl4·3H2O were used for the process of BG, and CS/Au/BG nanocomposite synthesis.
Bioactive Glass Synthesis
In this study only one composition of nano-bioactive glass (BG), with the composition (45% SiO2, 32.5% CaO, 15% Na2O, and 7.5% P2O5 wt%) was synthesized through the sol–gel process [40, 41]. TEOS has been put into a solution of nitric acid with a concentration of 2 M, and the mixture was agitated for half an hour. Following that, sodium phosphate and calcium hydroxide were each added to the solution at up for 30 min whereas the solution has been kept stirred. After stirring the newly acquired solution for a further hour, a transparent sol was produced. After that, the sol was allowed to gel before being dried. The BG powder was then annealed at 400 °C and collected the white powder as shown in Fig. S1 [40,41,42,43].
CS/Au/BG Nanocomposite Synthesis
Gamma radiation induced synthesis of CS/Au/BG nanocomposite as follows: 2.5 g of bioactive glass (BG) was ultrasonically suspended by stirring in 100 mL deionized water for 0.5 h. Then, 0.5 g of chitosan (CS) was dissolved in 100 mL 1% (v/v) aqueous acetic acid and stirred at 60 °C using a magnetic stirrer for 1 h until the polymer completely soluble.
After that, CS solution is added to bioactive glass solution and stirred for 0.5 h. Then, 5 ml of 0.027 M HAuCl4 was added to the solution and kept with continuous stirring at room temperature for about 1 h to insure well homogeneity of the solution.
Finally, the Au3+/chitosan/bioactive glass solutions were irradiated with 25.0 kGy using Co-60 γ-cell-220 sources (manufactured by the Atomic Energy Authority of India) with dose rate 1.1 kGy/h installed at the NCRRT, EAEA, Cairo, Egypt [44]. The color of the non-irradiated solution turned from light yellow into purple color with exposure to radiation. Finally, the gamma-irradiated solution was filtered and washed several times by distilled water and ethanol, then dried in air at 70 °C to collect the faint brown powder as shown in Fig. S1.
Characterization of CS/Au/BG Nanocomposite
Firstly, the stoichiometry of the CS/Au/BG nanocomposite are analyzed using the energy dispersive X-ray analysis spectrometer (EDX), JEOL JSM-5600 LV, Japan. Surface morphology of pure BG, and CS/Au/BG nanocomposite was determined by scanning electron microscope (SEM) ZEISS, EVO-MA10. X-ray diffraction procedure for the XRD spectra recorded by the X-ray diffractometer (model: Shimadzu XRD-6000) [45,46,47,48,49].
Antimicrobial Potential
The assay of agar-disc diffusion [50], was conducted to evaluate the antimicrobial potential of the synthesized bioactive glass (BG) and CS/Au/BG nanocomposite against some selected pathogenic unicellular fungi and bacteria.
The tested bacteria were grouped into Gram-positive as Bacillus subtilis, and Staphylococcus aureus, and Gram-negative as Proteus vulgaris, Pseudomonas aeruginosa, Escherichia coli, Proteus mirabilis, Salmonella typhi, and Klebsiella pneumoniae, and the tested unicellular fungi were Candida tropicalis, and Candida albicans. These microbial strains (clinical isolates) were obtained from the culture collection at Drug Microbiology Lab., Drug Radiation Research Department, NCRRT, EAEA, Cairo, Egypt. The tested bacteria were inoculated on nutrient agar for one day at 37 °C, while fungal strains were inoculated on malt extract agar (MEA) plates then incubated for 3–5 days at 28 ± 2 °C, and then kept at 4 °C for further use [51].
It must be mentioning that, the microbial inoculums of all the tested microbes must be fixed to a specific standard 0.5 McFarland, which fixed at 2 × 108 CFU/mL for the tested bacteria and unicellular fungi, and these standardization must be occurred before the microbial surface inoculation and ZOI measuring. Amoxicillin as a standard antibacterial agent, and nystatin as a standard antifungal positive control, must be included with the ZOI test to assess the antimicrobial potential of the synthesized samples [52]. Finally, to evaluate the ZOI potency, all Petri dishes must be incubated overnight at 37 °C and the antimicrobial potential was tested as the diameter of ZOI (mean ± standard error) of the tested samples [53].
Potential Antibiofilm Behavior
The antibiofilm potential of the synthesized bioactive glass (BG), and CS/Au/BG nanocomposite was assessed after conducting the assay of the test tube and tested against some selected pathogenic microbes (tested in ZOI assay), and the results were compared with the control non-treated samples, and finally the semi-qualitative assay regarding the microbial biofilm hindrance was conducted according to the method described by Christensen et al. [54].
It must be noted that, as in ZOI assay, the inoculums of the tested bacteria and unicellular fungi must be fixed according to 0.5 McFarland and adjusted at 2 × 108 CFU/mL, and then incubated overnight at 37 °C before the antibiofilm assay. Firstly, the antibiofilm test stated after mixing about 0.5 mL of the liquid nutrient broth (with the fixed microbes), and the tested samples in the designed test tubes, and incubated overnight at 37 °C. Secondly, after incubation, all the treated and non-treated tubes were dumped, and all the investigated tubes were cleaned with phosphate buffer saline (PBS; pH 7.0), and finally, washed several times with deionized water [55].
After that, the adhered microbial cells in the investigated tubes must be fixed with 3.5% sodium acetate (5 mL) for about 15 min and finally cleaned several times with deionized water. Finally, the cleaned tubes with the fixed microbial biofilm must be stained with 0.15% crystal violet (CV; 5 ml) for about 15 min to estimate the semi-qualitative antibiofilm activity of the synthesized samples. Finally, to determine the semi-quantitative antibiofilm potential of the synthesized samples, the CV stained microbial cells were dissolved by the action of ethanol solution (5 mL), and the O.D. of the dissolved CV was tested and counted after applying the UV–Vis. spectroscopy method at a fixed wavelength (570 nm), and the microbial biofilm inhibition % was estimated according to the subsequent Eq. (1) [54].
Growth Curve Assay
The method of Huang et al. [56], was conducted to estimate the effect of the synthesized bioactive glass (BG), and CS/Au/BG nanocomposite on S. aureus kinetic growth curve. Before testing, the inoculum of the tested bacteria was adjusted by standardized 0.5 McFarland in 2 × 108 CFU/mL, after that, the synthesized bioactive glass (BG) and CS/Au/BG nanocomposite mixed in the tested tube-containing the investigated bacteria. In the test protocol, the O.D. at fixed wavelength (600 nm) was measured after 2 h for about 24 h, and the final spectrum and relationship was conducted between the average of duplicate assignments and a time (hours) to get the standard growth curve, and assess the impact of the synthesized samples on the kinetic growth of the tested S. aureus.
UV Exposure Effect
The adjusted bacterial cells; according to standard 0.5 McFarland (2 × 108 CFU/mL), were mixed with the synthesized bioactive glass (BG) and CS/Au/BG nanocomposite. Following the mixing, all tubes were UV-irradiated, and the antimicrobial potency of the UV-irradiated samples were tested against the most sensitive S. aureus through the method of the optical density [57], and compared with the control non-UV irradiated samples.
The investigated tubes were classified into two conditions, tubes including non-UV irradiated bioactive glass (BG), and CS/Au/BG nanocomposite, and tubes with a bioactive glass (BG), and CS/Au/BG nanocomposite and UV-irradiated. They were exposures at various times (0, 15, 30, 45, 60, and 75 min). It must be noted that the treated samples were noted to form turbidity and were determined at fixed wavelength at 600 nm. The UV-lamp source was fixed on the tested samples at 37 °C (7.0 mW/cm2 disturbance). The method conducted by Abd Elkodous et al. [58] was used to determine the inhibition % after using the Eq. (1).
Protein Leakage Assay
To determine the estimated bacterial reaction mechanism of the synthesized samples, the assay of protein leakage was assessed. The bacterial inoculums were fixed by standard 0.5 McFarland (2 × 108 CFU/mL), and mixed with different concentrations of the synthesized samples.
Bioactive glass (BG), and CS/Au/BG nanocomposite-free broth mixed with culture were used as the negative control. After incubation at 37 °C for about 5 h, all the tested samples were separated by centrifugation for 20 min at 5000 rpm [59]. After centrifugation, the separated supernatant (100 μL) for the examined samples were mixed with the solution of Bradford reagent (1 mL), and O.D. at fixed wavelength (595 nm) was measured in the dark after incubation for 10 min at 37 °C [59].
SEM Imaging
To prepare the bacterial samples for the SEM imaging, the cells of S. aureus must be cleaned several times with PBS, and subsequently fixed with the solution of glutaraldehyde (3.5%). The fixed S. aureus cells were again washed several times with PBS and finally mixed with the solution of ethanol for 30 min at room temperature and then air-dried. The fixed bacterial cells (S. aureus) were placed on the aluminum stump to begin the SEM imaging [60]. The surface morphology and any changes in the treated and non-treated bacterial cells were investigated with the SEM imaging.
Statistical Analysis
The statistical analysis of the obtained results was investigated after applying the ONE WAY ANOVA (at P < 0.05) and Duncan’s methods [61]. SPSS software version 15 was conducted to examine the accepted results.
Results and Discussions
Structural Analyses
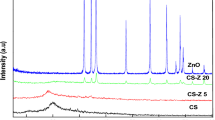
The X-ray diffraction (XRD) patterns for base bioactive glass BG and CS/Au/BG nanocomposite are shown in Fig. 1. The XRD patterns show the presence of crystalline phase of sodium calcium silicate [Na2Ca2Si3O9] (card no: PDF#01-10782 PDF#02-0961, Na2CaSi3O8 (card number: PDF#12-0671) [62, 63]. Kumar et al. [62], have also reported the appearance of both (Na2Ca2Si3O9 &Na2CaSi3O8) phases in the prepared bioactive glass–ceramic sample. Also, Ying Dou et al., have reported the presence of phase of Na2Ca2Si3O9 in XRD data [64]. Also, their diffraction peaks appeared in XRD pattern correspond to (111), (200), (220), and (311) crystal planes of Au NPs (JCPDS 01-1174) [65]. Further, the crystallite size of CS/Au/BG nanocomposite was determined via Scherer Equation and found in the range of 22.83 nm. XRD results confirmed the successful preparation of CS/Au/BG nanocomposite.
Surface morphology of the prepared base bioactive glass (BG) and CS/Au/BG nanocomposite are shown in Fig. 2. It can be recognized from Fig. 2a image that base bioactive glass presents as a conducted materials semi equal-distributed sample, and were regularly in a pure form. Similarly, Fig. 2b shows the corresponding SEM magnified image of base bioactive glass. Figure 2c displays the SEM validation of the synthesized CS/Au/BG nanocomposite, and appears as a hazy particles loaded with different elements (CS, and Au NPs) and both CS and Au NPs (bright particles) were distributed equally across BG nanocomposite. Finally, Fig. 2d shows the corresponding SEM magnified image of CS/Au/BG nanocomposite.
EDX spectroscopy was employed to analyze the elemental structure and to validate the prepared samples chemically [66]. The elemental composition of base bioactive glass (BG), and the synthesized CS/Au/BG nanocomposite was evaluated from EDX spectra as presented in Fig. 3. Figure 3a revealed the presence of all fundamental elements of base bioactive glass, including (Ca, P, Na, Si, and O), which is related to the BG sample. Figure 3b revealed the presence of all fundamental elements of CS/Au/BG nanocomposite, including (Ca, P, Na, Si, and O), which is related to the BG sample. Also, the carbon and nitrogen (C & N) elements that appear are correspond to CS polymer besides the Au element’s presence. EDX and XRD data confirmed the successful CS/Au/BG nanocomposite synthesis without any foreign elements or phases.
Furthermore, the uniform distribution of these elements over whole CS/Au/BG nanocomposite sample is proved via elemental mapping images as illustrated in Fig. 4. All images are identified as Si, P, Ca, C, O, Na, Au, and N for CS/Au/BG nanocomposite. From this figure, it is obvious that BG are similar in terms of the appearance of Ca, P, Na, Si, and O atoms, together they are homogenously distributed. Also, C, and N atoms were for Chitosan polymer, and Au atom for the synthesized loaded Au NPs.
Antimicrobial Activity
Across the time, the antibiotics and antiseptic agents were manufactured to control the microbial infectious diseases and after uncontrolled usage, the microbial resistance elevated and become useless [67]. To overcome the prolonged problem; new and novel green nanocomposites must be produced as an excellent antimicrobial agent to control the spread of microbial infectious diseases [68].
The selected agar-disc diffusion assay [69], was used to assess the antimicrobial ZOI behavior of the synthesized bioactive glass (BG), and CS/Au/BG nanocomposite at a specific concentration (10 µg/mL). After investigating the ZOI results, the synthesized samples are possessed the antimicrobial potential against the tested microbes and especially against both Staphylococcus aureus, and Escherichia coli. Particularly, the synthesized CS/Au/BG nanocomposite owned the most increased effect more than BG, as noted in Fig. 5 and tabulated in Table 1.
After the analysis of the result, it may be noted that the synthesized CS/Au/BG nanocomposite was more active against Gram-positive bacteria more than Gram-negative as compared with the published paper [70]. CS/Au/BG nanocomposite antimicrobial potency was compared with the positive controls (AX & NS), and the results obtained that the synthesized CS/Au/BG nanocomposite more active than the AX and NS. The sample purity, composition, and the incorporation with Au NPs may be important factors that evaluated the antimicrobial features. Basically, there is a relation between the physicochemical property of the synthesized nanocomposite and the ZOI results; the proper physical and chemical features, the nanoscale form, purity, and good distribution of the loaded Au NPs make the antimicrobial behavior of the synthesized sample more power than the synthetic disinfectants. It allows more interaction with the microbial cells, which intern increasing the contact and killing character [71]. In addition to an encouraged physicochemical feature of the synthesized nanocomposite, another elevated factor increasing the antimicrobial potential like strength and the stability of the synthesized samples, and the CS and Au incorporations [72].
Some of the suggested reaction mechanism according to the literature stated that the designed nanocomposites modify the bacterial surface appearance, and the cellular behavior, which finally alter the membrane permeability and create the oxidative pressure reply genes in the bacterial cell; because of hydrogen peroxide molecule formation. The most effected species was the reactive oxygen species (ROS) which diffused inside the treated bacteria cells which suggested to be the more effective reaction mechanism [73], finally, the strong connection between the nanoscale composite and microbial cells allows the elevated inhibition and killing of the pathogenic microbes.
Antibiofilm Potential
Some specific pathogenic microorganisms are identified for the potential for biofilm creation as exopolysaccharide molecules [55]. A tube method [74], was designed for the antibiofilm assessment of the synthesized bioactive glass (BG), and CS/Au/BG nanocomposite.
A negative result regarding biofilm repression of the tested S. aureus represented as air–liquid interface distinct whitish-yellow matt, and attached to the walls of designed tubes which staining with blue color after the treatment with CV. This noted negative results is exhibited in the absence of the synthesized bioactive glass (BG), and CS/Au/BG nanocomposite which serve as control. In difference, a positive result regarding the inhibition impact of the synthesized samples represented a faint blue color or the absence of any CV color. Additionally, bacterial ring development was limited, and the antibiofilm results of the synthesized CS/Au/BG nanocomposite more than the activity of bioactive glass (BG).
Semi-quantitative antibiofilm inhibition % is conducted by a method of UV–Vis. spectroscopy at fixed wavelength (570.0 nm). Detailed method is started after dissolving the stained microbial matt with ethanol and the O.D. is assessed to calculate the inhibition % by Eq. (1). The inhibition % is calculated and represented in Table 2 for the synthesized bioactive glass (BG), and CS/Au/BG nanocomposite. The most elevated inhibition of bioactive glass (BG), and CS/Au/BG nanocomposite percentage was inhibited for S. aureus (15.65%, and 77.24%), followed by C. albicans (13.32%, and 64.75%), and B. subtilis (16.09%, and 57.51%), respectively as described in Table 2, and Fig. S2.
The synthesized CS/Au/BG nanocomposite inhibits biofilm structure at its irreversible adhesion phase [74]. However, the automatic action of the synthesized CS/Au/BG nanocomposite upon biofilm structure has yet to be approved. The understanding of the inhibitory % might be determined by numerous factors, such as antimicrobial activity, physicochemical property, penetration abilities, and other chemical outcomes about the charge connection, metal NPs (Au) incorporation, and effective functional group in CS molecule [75]. It was noticed that the synthesized CS/Au/BG nanocomposite inhibited microbial growth and biofilm production. After controlling the exopolysaccharide secretion, the inhibition of the biofilm creation may be positively occurred [55].
Growth Curve Assay: Kinetic Study
The kinetic study regarding the effect of the synthesized bioactive glass (BG), and CS/Au/BG nanocomposite on the growth and multiplication of the tested S. aureus is exhibited in Fig. 6. The growth kinetic of the non-treated control S. aureus occurred normally, and measured O.D. value at specific wavelength (600 nm) reached 2.98 nm. While, bioactive glass (BG) produced a small differences with the control sample, and the O.D. was measured to be 2.01 nm. Positively, after CS/Au/BG nanocomposite addition, a notable influence on the kinetic growth curve was detected, and the measured O.D. was noted at 0.265 nm, indicating the promising inhibition effect on S. aureus growth kinetic. CS/Au/BG nanocomposite show additionally repressing potential more than bioactive glass (BG), and the superior effect was due to the catalytic potential of the antibacterial loaded Au NPs and CS [76].
On the surface of nanocomposites, the generation of ROS may occur as a lethal factor against bacterial cells [77, 78]. CS/Au/BG nanocomposite is specific for creating ROS, causing protein oxidation, bacterial DNA damage, and lipid peroxidation that can destroy the tested microbes. Additionally, the strong attraction between the synthesized nanocomposite and S. aureus membrane is due to the negative charge of the cells, the release of positive ions like (Au+), and the positive nature of the CS functional groups from the surface of the synthesized CS/Au/BG nanocomposite, and the gold ions released having a positive charge and increasing the lethal connection. Another fit reactivity was duo to the nano-size, stability, purity, surface charge, and chemical configuration of the synthesized CS/Au/BG nanocomposite, which could elevate the possibility to connect with the more pathogenic bacteria.
In the related paper, Xu et al. [79], reported that after nanocomposite treatment and UV-exposure for about 80 min, the membrane of E. coli was malformed, and the death of bacteria occurred. Another report [80], stated that, novel synthesized nanocomposites demonstrated strong antibacterial behaviors against the tested E. coli and S. aureus.
UV Exposure Effect
Figure 7 indicates that the antimicrobial potential of the synthesized nanocomposite strongly improved with the increasing UV exposure period, and conformed to the deactivation of the S. aureus cells following UV illumination. The favorable impacts on the extension and development of S. aureus were reported with the exhibit time. The UV assay results confirmed that, the growth of bacterial cells positively influenced following the UV illumination of the synthesized CS/Au/BG nanocomposite corresponded with the non-treated control, and the Au-, and CS-free bioactive glass (BG).
UV investigation results indicated that the growth of bacteria is influenced and decreases after the UV illumination of the synthesized nanocomposites. UV radiation raises the possibility for CS/Au/BG nanocomposite photo-activation more than BG. CS/Au/BG nanocomposite is a perfect biocidal agent after the excitation of UV irradiation.
Generally, most of the synthesized nanocomposites contributed to the formation of ROS following the acceptance of photons from the UV illumination process [81]. The created ROS (as hydrogen peroxide) after the UV excitation may be the promising factor for the bacterial biocidal process after the interaction with the bacterial membrane. Finally, the infusion of the created ROS within the bacterial cell caused the formation of stable hydroxyl free radical, and the death of the bacterial cell was noted [77, 78].
Protein Leakage Assay
In the protein leakage assay, the bacterial cell free supernatant has conducted for the determination of the quantity of bacterial proteins released from the bacterial cells. Figure 8, illustrated that the quantity of bacterial protein released from the cell is directly proportional to the increased concentrations of the synthesized bioactive glass (BG) and CS/Au/BG nanocomposite and was found to be 70.58 and 198.25 µg/ml, respectively at the concentration of 1.0 mg/mL. The present results presented the positive effect of the synthesized CS/Au/BG nanocomposite, and confirmed the catalytic nature as antibacterial agent. CS/Au/BG nanocomposite performs a noted spots around the tested bacterial cells and around S. aureus membrane, and the present facts confirmed the bleeding out of protein molecules from the membrane of S. aureus and approved their present in the bacterial cytoplasm.
The presented results indicated that the synthesized CS/Au/BG nanocomposite improved the bacterial membrane leakage and changed the permeability of the bacterial cell membrane more than bioactive glass (BG). Additionally, the bacterial death was due to the interference with the plasma membrane through the protein leakage after the treatment with the synthesized CS/Au/BG nanocomposite, which destroy the line defense of the bacterial cell.
From the literature point of view, recent articles as [82] and [83], represent the exact inhibition results following the usage of novel nanocomposites and define the mode of action according to the concentration-dependence which entirely effected the bacterial protein leakage and subsequently destroy the plasma membrane of some selected pathogenic bacteria. A positive report conducted by Paul et al. [84], verified that the exact determination of the protein leakage must be performed as a step for the reaction mechanism determination, and confirmed to be a critical method.
Bacterial SEM Imaging
The method of SEM imaging is considered the confirmatory test for the bacterial reaction mechanism determination, as stated in Fig. 9. The SEM imaging of the control S. aureus demonstrated the normal shape of the tested bacteria and normal number and conformed the normal surface morphology as displayed in Fig. 9a; inserted the magnified bacterial cell without any abnormalities.
Positively, by the treatment of the synthesized CS/Au/BG nanocomposite treatment, abnormalities were noted as the irregular shape of the S. aureus cells as noted in Fig. 9b. After the magnification of one treated cells (inserted in Fig. 9b), there are noted malformations as the semi-lysis of the external surface and deformations of the S. aureus cells. Additionally, the synthesized CS/Au/BG nanocomposite achieved the semi-complete lysis of the cells, and a noted decrease in the total viable count. The magnified cells in Fig. 9b confirms the formation of pits and spots along with the treated bacterial cell, which approved by the assay of protein leakage. Finally, we noted a layers of the synthesized CS/Au/BG nanocomposite across the treated cells which may be because of the charge attractions between positively charged Au+ and the positive function group in CS founded in the synthesized CS/Au/BG nanocomposite, and negatively charged bacterial cell.
From the literature point of view, the reaction mechanism and the inhibition potential of the synthesized nanocomposites may be due to the creation of stable ROS from the reactive Au NPs and CS loaded on the CS/Au/BG nanocomposite surface.
The synthesized CS/Au/BG nanocomposite may attach to the bacterial cells through the charge attraction which in turn altering the bacterial membrane and changing the microbial permeability due to the membrane break down. The response of bacterial cells seems in production of genes making an oxidative stress due to the positive action of the created ROS from the surface of the synthesized CS/Au/BG nanocomposite [69].
We recognize that the synthesized CS/Au/BG nanocomposite start the action after the adhesion to the microbial surface by the charge attraction, allowing the leakage of the bacterial membrane, which results in the formation of holes and cavities, and finally stops the ions transport from and inside the bacterial cells [85]. In response to the Au NPs and CS function group, ROS is created and penetrated inside the treated bacterial cell, with the response of harming the main micro organelles (like DNA, and plasmid). Finally, genotoxicity and cellular toxicity occurs due to the harmful effect and stress of the produced ROS [85].
Conclusion
In this study only one composition of nano-bioactive glass (BG), with the composition 45% SiO2, 32.5% CaO, 15% Na2O, and 7.5% P2O5 wt% was synthesized through the sol–gel process. Gamma radiation was applied at 25.0 kGy using Co-60 γ-cell-220 sources to induce the synthesis of CS/Au/BG nanocomposite. The XRD patterns show the presence of crystalline phase of sodium calcium silicate (Na2Ca2Si3O9, Na2CaSi3O8). Also, their diffraction peaks appeared in XRD pattern correspond to (111), (200), (220), and (311) crystal planes of Au NPs. XRD results confirmed the successful preparation of CS/Au/BG nanocomposite. The antimicrobial effectiveness of the synthesized BG and CS/Au/BG nanocomposite has been evaluated against a selection of pathogens (yeast and bacteria), and seem to be effective against some pathogenic bacteria (Staphylococcus aureus, and Escherichia coli). CS/Au/BG nanocomposite possessed the highest impact, against every pathogenic microbe that was examined. In growth curve assay, after adding BG, slight changes were detected, and OD600 was calculated to be 2.01 nm. The nanocomposite CS/Au/BG exhibited the lowest OD600 value (0.265 nm), demonstrating a repressive influence on the growth of S. aureus. In UV experiment, the bacterial growth finished at the lowest growth because of the deactivation after UV illumination. In protein leakage assay, the amount of bacterial protein removed is directly proportional after increasing the concentration of BG, and CS/Au/BG nanocomposite and counted to be 70.58, and 198.25 µg/ml following the treatment with 1.0 mg/mL BG, and CS/Au/BG nanocomposite, respectively, which describes the appearance of holes in the S. aureus membrane, and help in making the proteins bleed out from the S. aureus cytoplasm. The synthesized CS/Au/BG nanocomposite may be applied as a novel and promising factor against pathogenic microbes invading the environment, and human health and control the microbial pathogenesis as a new applicant in infectious disease, and wastewater treatments.
Data Availability
Not applicable.
References
S. Jagga, M.S. Hasnain and A.K. Nayak, Chitosan-based scaffolds in tissue engineering and regenerative medicine, in Chitosan in Biomedical Applications (Elsevier, Amsterdam, 2022), pp. 329–354.
T. Freyman, I. Yannas, and L. Gibson (2001). Cellular materials as porous scaffolds for tissue engineering. Prog. Mater. Sci. 46 (3–4), 273–282.
P. Abdollahiyan, F. Oroojalian, M. Hejazi, M. de la Guardia, and A. Mokhtarzadeh (2021). Nanotechnology, and scaffold implantation for the effective repair of injured organs: an overview on hard tissue engineering. J. Controll. Release 333, 391–417.
J. Yao, S. Radin, P. S. Leboy, and P. Ducheyne (2005). The effect of bioactive glass content on synthesis and bioactivity of composite poly (lactic-co-glycolic acid)/bioactive glass substrate for tissue engineering. Biomaterials 26 (14), 1935–1943.
T. Waltimo, T. Brunner, M. Vollenweider, W. J. Stark, and M. Zehnder (2007). Antimicrobial effect of nanometric bioactive glass 45S5. J. Dental Res. 86 (8), 754–757.
L. Drago, M. Toscano, and M. Bottagisio (2018). Recent evidence on bioactive glass antimicrobial and antibiofilm activity: a mini-review. Materials 11 (2), 326.
C. Wu and J. Chang (2014). Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Controll. Release 193, 282–295.
S. Naseri, W. C. Lepry, and S. N. Nazhat (2017). Bioactive glasses in wound healing: hope or hype? J. Mater. Chem. B 5 (31), 6167–6174.
S. Froum, S. C. Cho, E. Rosenberg, M. Rohrer, and D. Tarnow (2002). Histological comparison of healing extraction sockets implanted with bioactive glass or demineralized freeze-dried bone allograft: a pilot study. J. Periodontol. 73 (1), 94–102.
A. Balamurugan, G. Sockalingum, J. Michel, J. Fauré, V. Banchet, L. Wortham, S. Bouthors, D. Laurent-Maquin, and G. Balossier (2006). Synthesis and characterisation of sol gel derived bioactive glass for biomedical applications. Mater. Lett. 60 (29), 3752–3757.
T. Turunen, J. Peltola, H. Helenius, A. Yli-Urpo, and R. P. Happonen (1997). Bioactive glass and calcium carbonate granules as filler material around titanium and bioactive glass implants in the medullar space of the rabbit tibia. Clin. Oral Implants Res. 8 (2), 96–102.
M. Gopal, R. Gogoi, C. Srivastava, R. Kumar, P. K. Singh, K. K. Nair, S. Yadav, and A. Goswami (2011). Nanotechnology and its application in plant protection. Plant Pathol. India: Vision 2030, 224–232.
R. Luo, X. Zhou, Y. Xiu, and H. Wang (2018). Preparation of hierarchically mesoporous bioactive glass and immobilization of lysozyme. J. Sol-Gel Sci. Technol. 87 (3), 584–592.
C. Vichery and J.-M. Nedelec (2016). Bioactive glass nanoparticles: from synthesis to materials design for biomedical applications. Materials 9 (4), 288.
H. Iqbal and T. Keshavarz, The Challenge of Biocompatibility Evaluation of Biocomposites, Biomedical Composites. (Elsevier, Amsterdam, 2017), pp. 303–334.
D. Khvostenko, T. Hilton, J. Ferracane, J. Mitchell, and J. Kruzic (2016). Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dental Mater. 32 (1), 73–81.
M. Abdallah, C. Benoliel, D. Drider, P. Dhulster, and N.-E. Chihib (2014). Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol. 196 (7), 453–472.
L. Drago, C. Vassena, S. Fenu, E. D. Vecchi, V. Signori, R. D. Francesco, and C. L. Romanò (2014). In vitro antibiofilm activity of bioactive glass S53P4. Future Microbiol. 9 (5), 593–601.
D. M. Marques, V. D. C. Oliveira, M. T. Souza, E. D. Zanotto, J. P. M. Issa, and E. Watanabe (2020). Biomaterials for orthopedics: anti-biofilm activity of a new bioactive glass coating on titanium implants. Biofouling 36 (2), 234–244.
M. Galarraga-Vinueza, J. Mesquita-Guimarães, R. Magini, J. Souza, M. Fredel, and A. Boccaccini (2017). Anti-biofilm properties of bioactive glasses embedding organic active compounds. J. Biomed. Mater. Res. A 105 (2), 672–679.
F. Pishbin, V. Mourino, J. Gilchrist, D. McComb, S. Kreppel, V. Salih, M. Ryan, and A. R. Boccaccini (2013). Single-step electrochemical deposition of antimicrobial orthopaedic coatings based on a bioactive glass/chitosan/nano-silver composite system. Acta Biomater. 9 (7), 7469–7479.
J. Mota, N. Yu, S. G. Caridade, G. M. Luz, M. E. Gomes, R. L. Reis, J. A. Jansen, X. F. Walboomers, and J. F. Mano (2012). Chitosan/bioactive glass nanoparticle composite membranes for periodontal regeneration. Acta Biomater. 8 (11), 4173–4180.
V. Amendola and M. Meneghetti (2009). Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 11 (20), 3805–3821.
S. Ristig, D. Kozlova, W. Meyer-Zaika, and M. Epple (2014). An easy synthesis of autofluorescent alloyed silver–gold nanoparticles. J. Mater. Chem. B 2 (45), 7887–7895.
H. B. Ahmed, H. E. Emam, H. M. Mashaly, and M. Rehan (2018). Nanosilver leverage on reactive dyeing of cellulose fibers: color shading, color fastness and biocidal potentials. Carbohydr. Polym. 186, 310–320.
H. E. Emam (2019). Arabic gum as bio-synthesizer for Ag–Au bimetallic nanocomposite using seed-mediated growth technique and its biological efficacy. J. Polym. Environ. 27 (1), 210–223.
H. B. Ahmed and H. E. Emam (2019). Synergistic catalysis of monometallic (Ag, Au, Pd) and bimetallic (AgAu, AuPd) versus Trimetallic (Ag-Au-Pd) nanostructures effloresced via analogical techniques. J. Mol. Liq. 287, 110975.
P. J. Rivero, E. Ibañez, J. Goicoechea, A. Urrutia, I. R. Matias, and F. J. Arregui (2017). A self-referenced optical colorimetric sensor based on silver and gold nanoparticles for quantitative determination of hydrogen peroxide. Sens. Actuators B: Chem. 251, 624–631.
K. Sztandera, M. Gorzkiewicz, and B. Klajnert-Maculewicz (2018). Gold nanoparticles in cancer treatment. Mol. Pharm. 16 (1), 1–23.
M. Hoseinnejad, S. M. Jafari, and I. Katouzian (2018). Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications. Crit. Rev. Microbiol. 44 (2), 161–181.
D. Bociaga, P. Komorowski, D. Batory, W. Szymanski, A. Olejnik, K. Jastrzebski, and W. Jakubowski (2015). Silver-doped nanocomposite carbon coatings (Ag-DLC) for biomedical applications–physiochemical and biological evaluation. Appl. Surf. Sci. 355, 388–397.
T. D. Tran, M. T. Nguyen, H. V. Le, D. N. Nguyen, Q. D. Truong, and P. D. Tran (2018). Gold nanoparticles as an outstanding catalyst for the hydrogen evolution reaction. Chem. Commun. 54 (27), 3363–3366.
F. Liao, B. Jiang, W. Shen, Y. Chen, Y. Li, Y. Shen, K. Yin, and M. Shao (2019). Ir-Au bimetallic nanoparticle modified silicon nanowires with ultralow content of Ir for hydrogen evolution reaction. ChemCatChem 11 (8), 2126–2130.
P. Fakhri, H. Mahmood, B. Jaleh, and A. Pegoretti (2016). Improved electroactive phase content and dielectric properties of flexible PVDF nanocomposite films filled with Au-and Cu-doped graphene oxide hybrid nanofiller. Synth. Met. 220, 653–660.
P. Fakhri, M. R. Vaziri, B. Jaleh, and N. P. Shabestari (2015). Nonlocal nonlinear optical response of graphene oxide-Au nanoparticles dispersed in different solvents. J. Opt. 18 (1), 015502.
Y. Tan, Y. Liu, L. Kong, L. Kang, and F. Ran (2017). Supercapacitor electrode of nano-Co3O4 decorated with gold nanoparticles via in-situ reduction method. J. Power Sources 363, 1–8.
H. Daraee, A. Eatemadi, E. Abbasi, S. Fekri Aval, M. Kouhi, and A. Akbarzadeh (2016). Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 44 (1), 410–422.
T. Cele, P. Beukes, T. Beuvier, E. Chavez, M. Maaza, and A. Gibaud (2017). Radiolytic conversion of platinum, rhodium, osmium and palladium salts into metal coatings and metal nanoparticles. Johnson Matthey Technol. Rev. 61 (4), 279–289.
R. Sokary, M. N. Abu el-naga, M. Bekhit, and S. Atta (2021). A potential antibiofilm, antimicrobial and anticancer activities of chitosan capped gold nanoparticles prepared by γ–irradiation. Mater. Technol. 37, 1–10.
K. Zheng and A. R. Boccaccini (2017). Sol-gel processing of bioactive glass nanoparticles: a review. Adv. Colloid Interface Sci. 249, 363–373.
A. Moghanian, M. H. M. Tajer, M. Zohourfazeli, Z. Miri, and M. Yazdi (2021). Sol-gel derived silicate-based bioactive glass: studies of synergetic effect of zirconium and magnesium on structural and biological characteristics. J. Non-Cryst. Solids 554, 120613.
E. Piatti, E. Verné, and M. Miola (2022). Synthesis and characterization of sol-gel bioactive glass nanoparticles doped with boron and copper. Ceram. Int. 48 (10), 13706–13718.
P. P. Singh, K. Dixit, and N. Sinha (2022). A sol-gel based bioactive glass coating on laser textured 316L stainless steel substrate for enhanced biocompatability and anti-corrosion properties. Ceram. Int. 48 (13), 18704–18715.
M. I. A. Abdel Maksoud, M. M. Ghobashy, G. S. El-Sayyad, A. M. El-Khawaga, M. A. Elsayed, and A. H. Ashour (2021). Gamma irradiation-assisted synthesis of PANi/Ag/MoS2/LiCo0.5Fe2O4 nanocomposite: efficiency evaluation of photocatalytic bisphenol A degradation and microbial decontamination from wastewater. Opt. Mater. 119, 111396.
B. Alshahrani, H. I. ElSaeedy, S. Fares, A. H. Korna, H. A. Yakout, M. I. A. A. Maksoud, R. A. Fahim, M. Gobara, and A. H. Ashour (2021). The effect of Ce3+ doping on structural, optical, ferromagnetic resonance, and magnetic properties of ZnFe2O4 nanoparticles. J. Mater. Sci.: Mater. Electron. 32 (1), 780–797.
M. I. A. Abdel Maksoud, A. El-Ghandour, G. S. El-Sayyad, R. A. Fahim, A. H. El-Hanbaly, M. Bekhit, E. K. Abdel-Khalek, H. H. El-Bahnasawy, M. Abd Elkodous, A. H. Ashour, and A. S. Awed (2020). Unveiling the effect of Zn2+ substitution in enrichment of structural, magnetic, and dielectric properties of cobalt ferrite. J. Inorg. Organomet. Polym. Mater. 30 (9), 3709–3721.
A. El-ghandour, A. S. Awed, M. I. A. Abdel Maksoud, and M. A. Nasher (2019). 1,2-Dihydroxyanthraquinone: synthesis, and induced changes in the structural and optical properties of the nanostructured thin films due to γ-irradiation. Spectrochim. Acta A: Mol. Biomol. Spectrosc. 206, 466–473.
E. K. Abdel-Khalek, D. A. Rayan, A. A. Askar, M. I. A. A. Maksoud, and H. H. El-Bahnasawy (2021). Synthesis and characterization of SrFeO3-δ nanoparticles as antimicrobial agent. J. Sol-Gel Sci. Technol. 97 (1), 27–38.
E. M. Abou Hussein, M. I. A. Abdel Maksoud, R. A. Fahim, and A. S. Awed (2021). Unveiling the gamma irradiation effects on linear and nonlinear optical properties of CeO2–Na2O–SrO–B2O3 glass. Opt. Mater. 114, 111007.
E. Álvarez-Fernández, A. Cancelo, C. Díaz-Vega, R. Capita, and C. Alonso-Calleja (2013). Antimicrobial resistance in E. coli isolates from conventionally and organically reared poultry: a comparison of agar disc diffusion and Sensi Test Gram-negative methods. Food Control 30 (1), 227–234.
A. H. Hashem, A. M. A. Khalil, A. M. Reyad, and S. S. Salem (2021). Biomedical applications of mycosynthesized selenium nanoparticles using Penicillium expansum ATTC 36200. Biol. Trace Elem. Res. 199, 1–11.
A. I. El-Batal, H. G. Nada, R. R. El-Behery, M. Gobara, and G. S. El-Sayyad (2020). Nystatin-mediated bismuth oxide nano-drug synthesis using gamma rays for increasing the antimicrobial and antibiofilm activities against some pathogenic bacteria and Candida species. RSC Adv. 10 (16), 9274–9289.
A. H. Ashour, A. I. El-Batal, M. I. A. A. Maksoud, G. S. El-Sayyad, S. Labib, E. Abdeltwab, and M. M. El-Okr (2018). Antimicrobial activity of metal-substituted cobalt ferrite nanoparticles synthesized by sol–gel technique. Particuology 40, 141–151.
G. D. Christensen, W. A. Simpson, A. L. Bisno, and E. H. Beachey (1982). Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 37 (1), 318–326.
M. A. Ansari, H. M. Khan, A. A. Khan, S. S. Cameotra, and R. Pal (2014). Antibiofilm efficacy of silver nanoparticles against biofilm of extended spectrum β-lactamase isolates of Escherichia coli and Klebsiella pneumoniae. Appl. Nanosci. 4 (7), 859–868.
W. Huang, J.-Q. Wang, H.-Y. Song, Q. Zhang, and G.-F. Liu (2017). Chemical analysis and in vitro antimicrobial effects and mechanism of action of Trachyspermum copticum essential oil against Escherichia coli. Asian Pac. J. Trop. Med. 10 (7), 663–669.
M. Abd Elkodous, A. M. El-Khawaga, M. I. A. Abdel Maksoud, G. S. El-Sayyad, N. Alias, H. Abdelsalam, M. A. Ibrahim, M. A. Elsayed, G. Kawamura, Z. Lockman, W. K. Tan, and A. Matsuda (2022). Enhanced photocatalytic and antimicrobial performance of a multifunctional Cu-loaded nanocomposite under UV light: theoretical and experimental study. Nanoscale 14 (23), 8306–8317.
M. Abd Elkodous, G. S. El-Sayyad, S. M. Youssry, H. G. Nada, M. Gobara, M. A. Elsayed, A. M. El-Khawaga, G. Kawamura, W. K. Tan, and A. I. El-Batal (2020). Carbon-dot-loaded Co x Ni 1− x Fe 2 O 4; x= 0.9/SiO 2/TiO 2 nanocomposite with enhanced photocatalytic and antimicrobial potential: an engineered nanocomposite for wastewater treatment. Sci. Rep. 10 (1), 1–22.
H. Agarwal, A. Nakara, S. Menon, and V. Shanmugam (2019). Eco-friendly synthesis of zinc oxide nanoparticles using Cinnamomum tamala leaf extract and its promising effect towards the antibacterial activity. J. Drug Deliv. Sci. Technol. 53, 101212.
M. Relucenti, G. Familiari, O. Donfrancesco, M. Taurino, X. Li, R. Chen, M. Artini, R. Papa, and L. Selan (2021). Microscopy methods for biofilm imaging: focus on SEM and VP-SEM pros and cons. Biology 10 (1), 51.
A. M. Brown (2005). A new software for carrying out one-way ANOVA post hoc tests. Comput. Methods Progr. Biomed. 79 (1), 89–95.
V. K. Vyas, A. S. Kumar, A. Ali, S. Prasad, P. Srivastava, S. P. Mallick, M. Ershad, S. P. Singh, and R. Pyare (2016). Assessment of nickel oxide substituted bioactive glass-ceramic on in vitro bioactivity and mechanical properties. Boletín de la Sociedad Española de Cerámica y Vidrio 55 (6), 228–238.
S. Singh, G. Singh, N. Bala, and K. Aggarwal (2020). Characterization and preparation of Fe3O4 nanoparticles loaded bioglass-chitosan nanocomposite coating on Mg alloy and in vitro bioactivity assessment. Int. J. Biol. Macromol. 151, 519–528.
Y. Dou, S. Cai, X. Ye, G. Xu, K. Huang, X. Wang, and M. Ren (2013). 45S5 bioactive glass–ceramic coated AZ31 magnesium alloy with improved corrosion resistance. Surf. Coat. Technol. 228, 154–161.
A. Nasri, B. Jaleh, S. Khazalpour, M. Nasrollahzadeh, and M. Shokouhimehr (2020). Facile synthesis of graphitic carbon nitride/chitosan/Au nanocomposite: a catalyst for electrochemical hydrogen evolution. Int. J. Biol. Macromol. 164, 3012–3024.
J. Zou, P. Sanelle, K. A. Pettigrew, and S. M. Kauzlarich (2006). Size and spectroscopy of silicon nanoparticles prepared via reduction of SiCl4. J. Clust. Sci. 17 (4), 565–578.
A. Russell (2003). Biocide use and antibiotic resistance: the relevance of laboratory findings to clinical and environmental situations. Lancet Infect. Dis. 3 (12), 794–803.
T. Jin and Y. He (2011). Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. J. Nanopart. Res. 13 (12), 6877–6885.
M. Abd Elkodous, G. S. El-Sayyad, I. Y. Abdelrahman, H. S. El-Bastawisy, A. E. Mohamed, F. M. Mosallam, H. A. Nasser, M. Gobara, A. Baraka, M. A. Elsayed, and A. I. El-Batal (2019). Therapeutic and diagnostic potential of nanomaterials for enhanced biomedical applications. Colloids Surf. B: Biointerfaces 180, 411–428.
M. I. A. A. Maksoud, G. S. El-Sayyad, A. H. Ashour, A. I. El-Batal, M. A. Elsayed, M. Gobara, A. M. El-Khawaga, E. K. Abdel-Khalek, and M. M. El-Okr (2019). Antibacterial, antibiofilm, and photocatalytic activities of metals-substituted spinel cobalt ferrite nanoparticles. Microb. Pathog. 127, 144–158.
K. Karthik, M. M. Naik, M. Shashank, M. Vinuth, and V. Revathi (2019). Microwave-assisted ZrO 2 nanoparticles and its photocatalytic and antibacterial studies. J. Clust. Sci. 30 (2), 311–318.
K. Karthik, S. Dhanuskodi, C. Gobinath, S. Prabukumar, and S. Sivaramakrishnan (2019). Fabrication of MgO nanostructures and its efficient photocatalytic, antibacterial and anticancer performance. J. Photochem. Photobiol. B: Biol. 190, 8–20.
Z.-X. Tang and B.-F. Lv (2014). MgO nanoparticles as antibacterial agent: preparation and activity. Braz. J. Chem. Eng. 31 (3), 591–601.
C. Ashajyothi, K. H. Harish, N. Dubey, and R. K. Chandrakanth (2016). Antibiofilm activity of biogenic copper and zinc oxide nanoparticles-antimicrobials collegiate against multiple drug resistant bacteria: a nanoscale approach. J. Nanostruct. Chem. 6 (4), 329–341.
H.-J. Park, H. Y. Kim, S. Cha, C. H. Ahn, J. Roh, S. Park, S. Kim, K. Choi, J. Yi, and Y. Kim (2013). Removal characteristics of engineered nanoparticles by activated sludge. Chemosphere 92 (5), 524–528.
Y. Zhang, T. P. Shareena Dasari, H. Deng, and H. Yu (2015). Antimicrobial activity of gold nanoparticles and ionic gold. J. Environ. Sci. Health C 33 (3), 286–327.
R. M. Fathy and A. Y. Mahfouz (2004) Eco-friendly graphene oxide-based magnesium oxide nanocomposite synthesis using fungal fermented by-products and gamma rays for outstanding antimicrobial, antioxidant, and anticancer activities, J. Nanostruct. Chem., 1–21.
A. Joe, S.-H. Park, D.-J. Kim, Y.-J. Lee, K.-H. Jhee, Y. Sohn, and E.-S. Jang (2018). Antimicrobial activity of ZnO nanoplates and its Ag nanocomposites: Insight into an ROS-mediated antibacterial mechanism under UV light. J. Solid State Chem. 267, 124–133.
Y. Xu, Q. Liu, M. Xie, S. Huang, M. He, L. Huang, H. Xu, and H. Li (2018). Synthesis of zinc ferrite/silver iodide composite with enhanced photocatalytic antibacterial and pollutant degradation ability. J. Colloid Interface Sci. 528, 70–81.
I. Matai, A. Sachdev, P. Dubey, S. U. Kumar, B. Bhushan, and P. Gopinath (2014). Antibacterial activity and mechanism of Ag–ZnO nanocomposite on S. aureus and GFP-expressing antibiotic resistant E. coli. Colloids Surf. B: Biointerfaces 115, 359–367.
L. F. Gaunt, C. B. Beggs, and G. E. Georghiou (2006). Bactericidal action of the reactive species produced by gas-discharge nonthermal plasma at atmospheric pressure: a review. IEEE Trans. Plasma Sci. 34 (4), 1257–1269.
S. Rajesh, V. Dharanishanthi, and A. V. Kanna (2015). Antibacterial mechanism of biogenic silver nanoparticles of Lactobacillus acidophilus. J. Exp. Nanosci. 10 (15), 1143–1152.
Z. Azam, A. Ayaz, M. Younas, Z. Qureshi, B. Arshad, W. Zaman, F. Ullah, M. Q. Nasar, S. Bahadur, and M. M. Irfan (2020). Microbial synthesized cadmium oxide nanoparticles induce oxidative stress and protein leakage in bacterial cells. Microb. Pathog. 144, 104188.
D. Paul, S. Maiti, D. P. Sethi, and S. Neogi (2021). Bi-functional NiO-ZnO nanocomposite: synthesis, characterization, antibacterial and photo assisted degradation study. Adv. Powder Technol. 32 (1), 131–143.
M. A. Maksoud, G. S. El-Sayyad, A. M. El-Khawaga, M. Abd Elkodous, A. Abokhadra, M. A. Elsayed, M. Gobara, L. Soliman, H. El-Bahnasawy, and A. Ashour (2020). Nanostructured Mg substituted Mn-Zn ferrites: a magnetic recyclable catalyst for outstanding photocatalytic and antimicrobial potentials. J. Hazard. Mater. 399, 123000.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). None.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Not required.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Sayyad, G.S., Abdel Maksoud, M.I.A., Fahim, R.A. et al. Gamma Radiation Induced Synthesis of Novel Chitosan/Gold/Bioactive Glass Nanocomposite for Promising Antimicrobial, and Antibiofilm Activities. J Clust Sci 34, 1877–1891 (2023). https://doi.org/10.1007/s10876-022-02357-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02357-9