Abstract
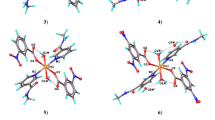
The synthesis, spectral characterization and crystal structure of two new nitrobenzoatocopper(II) complexes, namely, [Cu2(2-O2Nbz)4(nia)2]·ACN (1) and [Cu2(2-O2Nbz)4(ACN)2] (2) (where 2-O2Nbz = 2-nitrobenzoate, nia = nicotinamide and ACN = acetonitrile) are reported. The complexes 1 and 2 form dinuclear units of the paddle-wheel type around the crystallographic inversion centers. The copper ions are bridged by four 2-nitrobenzoate anions and the neutral N-donor ligands, viz. nicotinamide in 1 and acetonitrile in 2, are coordinated at apical positions. Selected geometric parameters of both complexes are compared with values for related tetra-2-nitrobenzoate complexes of copper(II) as well as the other dimeric copper(II) carboxylates with apical nicotinamide and acetonitrile ligands. The molecules of 1 are linked with N–H···O and C–H···O hydrogen bonds. The π–π stacking interactions in 1 are observed between benzene rings of 2-nitrobenzoate anions and pyridine rings of nicotinamide and also between acetonitrile molecules and benzene rings of 2-nitrobenzoate anions. The C–H···O hydrogen-bonds and CH/π interactions are observed in crystal structure of 2.
Graphical Abstract
The synthesis, spectral characterization and crystal structure of two new nitrobenzoatocopper(II) complexes, namely, [Cu2(2-O2Nbz)4(nia)2]·ACN and [Cu2(2-O2Nbz)4(ACN)2] (where 2-O2Nbz = 2-nitrobenzoate, nia = nicotinamide and ACN = acetonitrile) are reported. The both complexes form dinuclear units of the paddle-wheel type around the crystallographic inversion centers.







Similar content being viewed by others
References
Desiraju GR (2007) Angew Chem Int Ed 46:8342
Brammer L (2004) Chem Soc Rev 33:476
Beatty AM (2003) Coord Chem Rev 246:131
Beatty AM (2001) CrystEngComm 3:243
Burrows AD (2004) Structure Bonding 108:55
Desiraju GR (2000) J Chem Soc Dalton Trans 3745
Stachová P, Valigura D, Koman M, Melník M, Korabik M, Mrozinski J, Głowiak T (2004) Polyhedron 23:1303
Moncol J, Kavalírová J, Lis T, Valigura D (2006) Acta Crystallogr E62:m3217
Klinga M, Sundberg MR, Melník M, Mrozinski J (1989) Inorg Chim Acta 162:39
Deutsch Z, Rubin-Preminger JM, Bernstein J, Becker JY (2006) CrystEngComm 8:41
Karmakar A, Bania K, Baruah AM, Baruah JB (2007) Inorg Chem Commun 10:959
Kavalírová J, Korabik M, Stachová P, Moncol J, Sillanpää R, Lis T, Mikloš D, Melník M, Mroziński J, Valigura D (2008) Polyhedron 27:1333
Kawata T, Uekusa H, Ohba S, Furukawa T, Tokii T, Muto Y, Kato M (1992) Acta Crystallogr B48:253
Kozlevčar B, Leban I, Turel I, Šegedin P, Petrič M, Pohleven F, White AJP, Williams DJ, Sieler J (1999) Polyhedron 18:755
Tsintsadze GV, Kiguradze RA, Shnulin AN, Mamedov KS (1984) J Struct Chem 25:911
Smolander K, Macko M, Valko M, Melník M (1992) Acta Chem Scand 46:29
Antsyshkina AS, Koksharova TV, Sadikov GG, Gritsenko IS, Sergienko VS, Egorova OA (2006) Russ J Inorg Chem 51:901
Kozlevčar B, Lah N, Turel I, Šegedin P, Petrič M, Pohleven F, White AJP, Williams DJ, Giester G (1999) Croat Chem Acta 72:427
Kozlevčar B, Baškovič P, Arko A, Golobič A, Kitanovski N, Šegedin P (2008) Z Naturforsch 63b:481
Bernstein J, Davis RE, Shimoni L, Chang NL (1995) Angew Chem Int Ed Engl 34:1555
Janiak C (2000) J Chem Soc Dalton Trans 3885
Karpova EV, Boltalin AI, Korenev YM, Zakharov MA, Trovanov SI (2001) Russ J Coord Chem 27:286
Ueyama N, Yamada Y, Takeda J, Okamura T, Mori W, Nakamura A (1996) Chem Commun 1377
Lah N, Giester G, Šegedin P, Murc A, Podlipnik K, Leban I (2001) Acta Crystallogr C57:546
Kögerler P, Williams PAM, Parajón-Costa BS, Baran EJ, Lezama L, Rojo T, Müller A (1998) Inorg Chim Acta 268:239
Agterberg FPW, Provo Kluit HAJ, Driessen WL, Reedijk J, Oevering H, Buijs W, Veldman N, Lakin MT, Spek AL (1998) Inorg Chim Acta 267:183
Jiang GQ, Li YZ, Wang SN, Li FF, Xu ZJ, Bai JF (2005) Acta Crystallogr E61:m1517
Calderazzo F, Donati N, Englert U, Marchetti F, Pampaloni G, Passarelli V (2003) Inorg Chim Acta 346:100
Morgan YR, Turner P, Kennedy BJ, Hambley TW, Lay PA, Biffin JR, Regtop HL, Warwick B (2001) Inorg Chim Acta 324:150
Papaefstathiou GS, Darrow BG, MacGillivray LR (2002) J Chem Crystallogr 32:191
Karpova EV, Boltalin AI, Zakharov MA, Sorokina NI, Korenev YM, Troyanov SI (1998) Z Anorg Allg Chem 624:741
Ghassemzadeh M, Aghapoor K, Neumuller B (1998) Z Naturforsch 53b:774
Suezawa H, Yoshida T, Umezawa Y, Tsuboyama S, Nishio M (2002) Eur J Inorg Chem 3148
Nakamoto K (1997) Infrared and raman spectra of inorganic and coordination compounds. Part B. Wiley, New York, p 60
Wasserman E, Snyder LC, Yager WA (1964) J Chem Phys 41:1763
Wasson JR, Shyr CI, Trapp C (1968) Inorg Chem 7:469
Melnik M (1982) Coord Chem Rev 42:259
Nonius (1998) COLLECT Nonius BV. Delft, The Netherlands
Otwinowski Z, Minor W (1997) Methods in enzymology. In: Carter CW Jr, Sweet RM (eds) Macromolecular crystallography, Part A, vol 276. Academic Press, New York, pp 307–326
Blessing RH (1995) Acta Crystallogr A51:33
Sheldrick GM (2008) Acta Crystallogr A64:112
Acknowledgments
We thank the Scientific Grant Agency of the Ministry of Education of Slovak Republic and the Slovak Academy of Sciences for financial support (1/0575/08 and 1/4454/07).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moncol, J., Vasková, Z., Stachová, P. et al. Self-Assembled Hydrogen-Bonding Chains of Copper(II) 2-Nitrobenzoate with Nicotinamide. J Chem Crystallogr 40, 179–184 (2010). https://doi.org/10.1007/s10870-009-9631-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-009-9631-z