Abstract
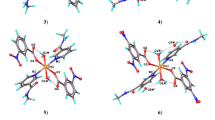
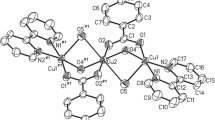
Nicotinamide was employed as a supramolecular reagent in the synthesis of six new copper(II) bromo-, iodo-, fluoro- and dibromobenzoate complexes. Structures of [Cu(2-Brbz)2(nia)2(H2O)2] (I), [Cu(2-Ibz)2(nia)2(H2O)2] (II), [Cu(2-Fbz)2(nia)2(H2O)2] (III), [Cu(4-Brbz)2(nia)2(H2O)2] (IV), [Cu(3,5-Br2bz)2(nia)2(H2O)2] (V), [Cu(F-Fbz)2(nia)2(H2O)] · H2O (VI) (nia = nicotinamide, 2-Brbz = 2-bromobenzoate, 4-Brbz = 4-bromobenzoate, 3,5-Br2bz = 3,5-dibromobenzoate, 2-Ibz = 2-iodobenzoate, 2-Fbz = 2-fluorobenzoate, 4-Fbz = 4-fluorobenzoate) were determined using X-ray analysis. Compound [Cu(2-Brbz)2(nia)2] · 2H2O (VII) was prepared by a new method and also characterized by X-ray powder diffraction and EPR spectroscopy. Compounds I–V are monomeric complexes with a square-bipyramidal coordination sphere around the Cu2+ ion. Complex VI is monomeric with coordination environment around the Cu2+ ion of a tetragonal-pyramid. Complexes I and VII present examples of coordination isomerism. Molecules of all compounds are connected by N—H⋯O and O—H⋯O hydrogen bonds from the NH2 groups of nicotinamide and water molecules which create supramolecular hydrogen-bonding-coordination chains and networks.
Similar content being viewed by others
References
Aakeröy, C. B., Schultheiss, N., & Desper, J. (2005). Directed supramolecular assembly of infinite 1-D M(II)-containing chains (M = Cu, Co, Ni) using structurally bifunctional ligands. Inorganic Chemistry, 44, 4983–4991. DOI: 10.1021/ic048405y.
Aakeröy, C. B., Desper, J., Levin, B., & Valdés-Martínez, J. (2006). Robust building blocks for inorganic crystal engineering. Inorganica Chimica Acta, 359, 1255–1262. DOI: 10.1016/j.ica.2005.09.051.
Aakeröy, C. B., Scott, B. M. T., Smith, M. M., Urbina, J. F., & Desper, J. (2009). Establishing amide⋯amide reliability and synthon transferability in the supramolecular assembly of metal-containing one-dimensional architectures. Inorganic Chemistry, 48, 4052–4061. DOI: 10.1021/ic801992t.
Addison, A. W., Rao, T. N., Reedijk, J., van Rijn, J., & Verchoor, G. C. (1984). Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[l,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. Journal of the Chemical Society, Dalton Transactions, 1984, 1349–1356. DOI: 10.1039/dt9840001349.
Arıcı, M., Yeşilel, O. Z., SŞhin, O., & Büyükgüngör, O. (2014). Two dimensional coordination polymers with 3,3′-thiodipropionate: An unprecedented coordination mode and strong hydrogen-bond network. Polyhedron, 67, 456–463. DOI: 10.1016/j.poly.2013.09.040.
Beatty, A. M. (2001). Hydrogen bonded networks of coordination complexes. CrystEngComm, 3, 243–255. DOI: 10.1039/b109127c.
Beatty, A. M. (2003). Open-framework coordination complexes from hydrogen-bonded networks: toward host/guest complexes. Coordination Chemistry Reviews, 246, 131–143. DOI: 10.1016/s0010-8545(03)00120-6.
Beobide, G., Castillo, O., Cepeda, J., Luque, A., Pérez-Yáñez, S., Román, P., & Thomas-Gipson, J. (2013). Metalcarboxylato-nucleobase systems: From supramolecular assemblies to 3D porous materials. Coordination Chemistry Reviews, 257, 2716–2736. DOI: 10.1016/j.ccr.2013.03.011.
Bernstein, J., Davis, R. E., Shimoni, L., & Chang, N. L. (1995). Patterns in hydrogen bonding: Functionality and graph set analysis in crystals. Angewandte Chemie Internation Edition in English, 34, 1555–1573. DOI: 10.1002/anie.199515551.
Betteridge, P. W., Carruthers, J. R., Cooper, R. I., Prout, K., & Watkin, D. J. (2003). CRYSTALS version 12: software for guided crystal structure analysis. Journal of Applied Crystallography, 36, 1487. DOI: 10.1107/s0021889803021800.
Boultif, A., & Lour, D. (2004). Powder pattern indexing with the dichotomy method. Journal of Applied Crystallography, 37, 724–731. DOI: 10.1107/s0021889804014876.
Burla, M. C., Caliandro, R., Camalli, M., Carrozzini, B., Cascarano, G. L., Giacovazzo, C., Mallamo, M., Mazzone, A., Polidori G., & Spagna, R. (2012). SIR2011: a new package for crystal structure determination and refinement. Journal of Applied Crystallography, 45, 357–361. DOI: 10.1107/s0021889812001124.
Ɖaković, M., Popović, Z., Giester, G., & Rajić-Linarić, M. (2008). Synthesis, spectroscopic and structural investigation of Zn(NCS)2(nicotinamide)2 and [Hg(SCN)2(nicotinamide)]n. Polyhedron, 27, 465–472. DOI: 10.1016/j.poly.2007.09.036.
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., & Puschmann, H. (2009). OLEX2: a complete structure solution, refinement and analysis program. Journal of Applied Crystallography, 42, 339–341. DOI: 10.1107/s0021889808042726.
Favre-Nicolin, V., & Černý, R. (2002). FOX, ‘free objects for crystallography’: a modular approach to ab initio structure determination from powder diffraction. Journal of Applied Crystallography, 35, 734–743. DOI: 10.1107/s0021889802015236.
Friš čić, T., Meštrović, E., Šklaec Šamec, D., Kaitner, B., & Fábián, L. (2009). One-pot mechanosynthesis with three levels of molecular self-assembly: Coordination bonds, hydrogen bonds and host-guest inclusion. Chemistry — A European Journal, 15, 12644–12652. DOI: 10.1002/chem.200901058.
Gör, K., Kürkçüoğlu, G. S., Yeşilel, O. Z., & Büyükgüngör, O. (2014). One-dimensional bimetallic cyano complexes with nicotinamide and isonicotinamide ligands. Journal of Molecular Structure, 1060, 166–175. DOI: 10.1016/j.molstruc.2013.12.024.
Halaška, J., Pevec, A., Strauch, P., Kozlevčar, B., Koman, M., & Moncol, J. (2013). Supramolecular hydrogen-bonding networks constructed from copper(II) chlorobenzoates with nicotinamide: Structure and EPR. Polyhedron, 61, 20–26. DOI: 10.1016/j.poly.2013.05.032.
James, S. L. (2003). Metal-organic frameworks. Chemical Society Reviews, 32, 276–288. DOI: 10.1039/b200393g.
Janiak, C. (2000). A critical account on π-π stacking in metal complexes with aromatic nitrogen-containing ligands. Journal of the Chemical Society, Dalton Transactions, 2000, 3885–3896. DOI: 10.1039/b003010o.
Janiak, C. (2003). Engineering coordination polymers towards applications. Dalton Transactions, 2003, 2781–2804. DOI: 10.1039/b305705b.
Kitagawa, S., & Uemura, K. (2005). Dynamic porous properties of coordination polymers inspired by hydrogen bonds. Chemical Society Reviews, 34, 109–119. DOI: 10.1039/b313997m.
Koksharova, T. V., Gritsenko, I. S., & Stoyanova, I. V. (2007). Coordination compounds of 3d-metal valerates and benzoates with nicotinamide. Russian Journal of General Chemistry, 77, 1635–1642. DOI: 10.1134/s107036320709023x.
Kose, D. A., Necefoğlu, H., SŞhin, O., & Büyükgüngör, O. (2011). Synthesis, spectral, thermal and structural study of monoaquabis(acetylsalicylato-κO)bis(nicotinamide-κN)copper(II). Journal of Chemical Crystallography, 41, 297–305. DOI: 10.1007/s10870-010-9876-6.
Lever, A. B. P. (1984). Inorganic electronic spectroscopy (2nd ed.) (Series: Studies in physical and theoretical chemistry, Vol. 33). Amsterdam, The Netherlands: Elsevier.
Ma, Z., & Moulton, B. (2007a). Mixed-ligand coordination species: A promising approach for “second-generation” drug development. Crystal Growth & Design, 7, 196–198. DOI: 10.1021/cg060602f.
Ma, Z., & Moulton, B. (2007b). Supramolecular medicinal chemistry: Mixed-ligand coordination complexes. Molecular Pharmaceutics, 4, 373–385. DOI: 10.1021/mp070013k.
Moncol, J., Maroszova, J., Koman, M., Melnik, M., Valko, M., Mazur, M., & Lis, T. (2008). Self-assembly of hydrogen-bonded supramolecular structures of two copper(II) 2-bromobenzoate complexes with 4-pyridylmethanol and nicotinamide. Journal of Coordination Chemistry, 61, 3740–3752. DOI: 10.1080/00958970802146031.
Moncol, J., Kuchtanin, V., Polakovičová, P., Mroziński, J., Kalińska, B., Koman, M., Padělková, Z., Segľa, P., & Melník, M. (2012). Study of copper(II) thiophenecarboxylate complexes with nicotinamide. Polyhedron, 45, 94–102. DOI: 10.1016/j.poly.2012.07.069.
Nakamoto, K. (1997). Infrared and Raman spectra of inorganic and coordination compounds (5th ed.) (Part B: Applications in coordination, organometallic, and bioinorganic chemistry, pp. 23, 56, 79). Chichester, UK: Wiley.
Pasán, J., Sanchiz, J., Lloret, F., Julve, M., & Ruiz-Pérez, C. (2011). Copper(II)-phenylmalonate complexes with the bi-functional ligands nicotinamide and isonicotinamide. Polyhedron, 30, 2451–2458. DOI: 10.1016/j.poly.2011.06.006.
Perec, M., Baggio, R. F., Peña, O., Sartoris, R. P., & Calvo, R. (2011). Synthesis and structures of four new compounds of the copper(II)-carboxylate-pyridinecarboxamide system. Inorganica Chimica Acta, 373, 117–123. DOI: 10.1016/j.ica.2011.03.065.
Petříček, V., Dušek, M., & Palatinus, L. (2014). Crystallographic computing system JANA2006: General features. Zeitschrift für Kristallographie — Crystalline Materials, 229, 345–352. DOI: 10.1515/zkri-2014-1737.
Shan, N., Hawxwell, S. M., Adams, H., Brammer, L., & Thomas, J. A. (2008). Self-assembly of electriactive thiacrown ruthenium(II) complexes into hydrogen-bonded chain and tape networks. Inorganic Chemistry, 47, 11551–11560. DOI: 10.1021/ic8007359.
Sheldrick, G. M. (2008). A short history of SHELX. Acta Crystallographica Section A, A64, 112–122. DOI: 10.1107/s0108767307043930.
Sheldrick, G. M. (2015a). SHELXT — Integrated space-group and crystal-structure determination. Acta Crystallographica Section A, A71, 3–8. DOI: 10.1107/s2053273314026370.
Sheldrick, G. M. (2015b). Crystal structure refinement with SHELXL. Acta Crystallographica Section C, C71, 3–8. DOI: 10.1107/s2053229614024218.
Smart, P., Bejarano-Villafuerte, A., & Brammer, L. (2013). Coordination chemistry meets halogen bonding and hydrogen bonding: building networks from 3-iodobenzoate paddlewheel units [Cu2(3-Ibz)4(L)2]. CrystEngComm, 15, 3151–3159. DOI: 10.1039/c3ce26890j.
Spek, A. L. (2015). PLATON SQUEEZE: a tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallographica Section C, C71, 9–18. DOI: 10.1107/s2053229614024929.
Stachová, P., Melník, M., Korabik, M., Mrozinski, J., Koman, M., Glowiak, T., & Valigura, D. (2007). Synthesis, spectral and magnetical characterization of monomeric [Cu(2-NO2bz)2(nia)2(H2O)2] and structural analysis of similar [Cu(RCOO)2(L-N)2(H2O)2] complexes. Inorganica Chimica Acta, 360, 1517–1522. DOI: 10.1016/j.ica.2006.08.019.
Xue, J., Hua, X., Yang, L., Li, W., Xu, Y., Zhao, G., Zhang, G., Liu, L., Liu, K., Chen, J., & Wu, J. (2014). Cobalt(II) and strontium(II) complexes of three isomers, nicotinamide, isonicotinamide and picolinamide. Journal of Molecular Structure, 1059, 108–117. DOI: 10.1016/j.molstruc.2013.11.001.
Valigura, D., Moncol, J., Korabik, M., Púčeková, Z., Lis, T., Mroziński, M., & Melník, M. (2006). New dimeric copper(II) complex [Cu(5-MeOsal)2(μ-nia)(H2O)]2 with magnetic exchange interactions through H-bonds. European Journal of Inorganic Chemistry, 2006, 3813–3817. DOI: 10.1002/ejic.200600477.
Vasková, Z., Moncol, J., Korabik, M., Valigura, D., Švorec, J., Lis, T., Valko, M., & Melník, M. (2010). Supramolecular dimer formation through hydrogen bond extensions of carboxylate ligands — Path for magnetic exchange. Polyhedron, 29, 154–163. DOI: 10.1016/j.poly.2009.06.056.
Vasková, Z., Kitanovski, N., Jagličić, Z., Strauch, P., Růžičková, Z., Valigura, D., Koman, M., Kozlevčar, B., & Moncol, J. (2014). Synthesis and magneto-structural characterization of copper(II) nitrobenzoate complexes containing nicotinamide or methylnicotinamide ligands. Polyhedron, 81, 555–563. DOI: 10.1016/j.poly.2014.07.017.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Halaška, J., Čechová, D., Lawson, M.K. et al. Self-assembly hydrogen-bonded supramolecular arrays from copper(II) halogenobenzoates with nicotinamide: Structure and EPR spectra. Chem. Pap. 70, 101–113 (2016). https://doi.org/10.1515/chempap-2015-0203
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2015-0203