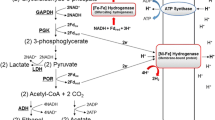
Abstract
Escherichia coli has four [NiFe]-hydrogenases (Hyd); three of these, Hyd-1, Hyd-2 and Hyd-3 have been characterized well. In this study the requirement for the F0F1-ATP synthase for the activities of the hydrogen-oxidizing hydrogenases Hyd-1 and Hyd-2 was examined. During fermentative growth on glucose at pH 7.5 an E. coli F0F1-ATP synthase mutant (DK8) lacked hydrogenase activity. At pH 5.5 hydrogenase activity was only 20% that of the wild type. Using in-gel activity staining, it could be demonstrated that both Hyd-1 and Hyd-2 were essentially inactive at these pHs, indicating that the residual activity at pH 5.5 was due to the hydrogen-evolving Hyd-3 enzyme. During fermentative growth in the presence of glycerol, hydrogenase activity in the mutant was highest at pH 7.5 attaining a value of 0.76 U/mg, or ~50% of wild type activity, and Hyd-2 was only partially active at this pH, while Hyd-1 was inactive. Essentially no hydrogenase activity was measured at pH 5.5 during growth with glycerol. At this pH the mutant had a hydrogenase activity that was maximally only ~10% of wild type activity with either carbon substrate but a weak activity of both Hyd-1 and Hyd-2 could be detected. Taken together, these results demonstrate for the first time that the activity of the hydrogen-oxidizing hydrogenases in E. coli depends on an active F0F1-ATP synthase during growth at high and low pH.
Similar content being viewed by others
References
Andrews SG, Berks BC, McClay J, Ambler A, Quail AM, Golby P, Guest R (1997) A 12-cistron Escherichia coli operon (hyf) encoding a putative proton-translocating formate hydrogenlyase system. Microbiology 143:3633–3647
Bagramyan KA, Martirosov SM (1989) Formation of an ion transport supercomplex in Escherichia coli. An experimental model of direct transduction of energy. FEBS Lett 246:149–152
Bagramyan K, Mnatsakanyan N, Poladian A, Vassilian A, Trchounian A (2002) The roles of hydrogenases 3 and 4, and the F0F1-ATPase, in H2 production by Escherichia coli at alkaline and acidic pH. FEBS Lett 516:172–178
Ballantine SP, Boxer DH (1985) Nickel-containing hydrogenase isoenzymes from anaerobically grown Escherichia coli K-12. J Bacteriol 163:454–459
Blbulyan S, Avagyan A, Poladyan A, Trchounian A (2011) Role of Escherichia coli different hydrogenases in H+ efflux and the FOF1-ATPase activity during glycerol fermentation at different pH. Biosci Rep 31:179–184
De Graef MR, Alexeeva S, Snoep JL, Teixeira MJ (1999) The steady-state internal redox state (NADH/NAD+) reflects the external redox state and is correlated with catabolic adaptation in Escherichia coli. J Bacteriol 184:2351–2357
Dharmadi Y, Murarka A, Gonsalez R (2006) Anaerobic fermentation of glycerol by Escherichia coli: A new platform for metabolic engineering. Biotechnol Bioeng 94:821–829
Gabrielyan L, Trchounian A (2009) Relationship between molecular hydrogen production, proton transport and the F0F1-ATPase activity in Rhodobacter sphaeroides strains from mineral springs. Int J Hydrogen Energy 34:2567–2572
Gonzalez R, Murarka A, Dharmadi Y, Yasdani SS (2008) A new model for the anaerobic fermentation of glycerol in enteric bacteria: trunk and auxiliary pathways in Escherichia coli. Metab Eng 10:234–245
Hu H, Wood TK (2010) An evolved Escherichia coli strain for producing hydrogen and ethanol from glycerol. Biochem Biophys Res Commun 391:1033–1038
Kim YL, Lee HS, Kim ES, Bae SS, Lim JK, Matsumi R, Lebedinsky AV, Sokolova TG, Kozhevnikova DA, Cha SS, Kim SJ, Kwon KK, Imanaka T, Atomi H, Bonch-Osmolovskaya EA, Lee JH, Kang SG (2010) Formate-driven growth coupled with H2 production. Nature 467:352–355
King PW, Przybyla AE (1999) Response of hya expression to external pH in Escherichia coli. J Bacteriol 181:5250–5256
Kirakosyan G, Trchounian K, Vardanyan Z, Trchounian A (2008) Copper (II) ions affect Escherichia coli membrane vesicles’ SH-groups and a disulfide-dithiol interchange between membrane proteins. Cell Biochem Biophys 51:45–50
Klionsky DJ, Brusiow WSA, Simoni R (1984) In vivo evidence for the role of the ε subunit as an inhibitor of the proton-translocating ATPase of Escherichia coli. J Bacteriol 160:1055–1060
Laurinavichene TV, Zorin NA, Tsygankov AA (2002) Effect of redox potential on activity of hydrogenase 1 and hydrogenase 2 in Escherichia coli. Arch Microbiol 178:437–442
Lowry OH, Rosebrough NJ, Farr AL, Randall RG (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lukey MJ, Parkin A, Roessler MM, Murphy BJ, Harmer J, Palmer T, Sargent F, Armstrong FA (2010) How Escherichia coli is equipped to oxidize hydrogen under different redox conditions. J Biol Chem 285:3928–3938
Menon NK, Robbins J, Wendt JC, Shanmugan KT, Przybyla AE (1991) Mutational analysis and characterization of the Escherichia coli hya operon, which encodes [NiFe] hydrogenase 1. J Bacteriol 173:4851–4861
Mnatsakanyan N, Bagramyan K, Vassilian A, Nakamoto RK, Trchounian A (2002) F0 cysteine, bCys21, in the Escherichia coli ATP synthase is involved in regulation of potassium uptake and molecular hydrogen production in anaerobic conditions. Biosci Rep 22:421–430
Mnatsakanyan N, Bagramyan K, Trchounian A (2004) Hydrogenase 3 but not hydrogenase 4 is major in hydrogen gas production by Escherichia coli formate hydrogenlyase at acidic pH and in the presence of external formate. Cell Biochem Biophys 41:357–366
Noda S, Takezawa Y, Mizutani T, Asakura T, Nishiumi E, Onoe K, Wada M, Tomita F, Matsushita K, Yokota A (2006) Alterations of cellular physiology in Escherichia coli in response to oxidative phosphorylation impaired by defective F1-ATPase. J Bacteriol 188:6869–6876
Redwood MD, Mikheenko IP, Sargent F, Macaskie LE (2008) Dissecting the roles of Escherichia coli hydrogenases in biohydrogen production. FEMS Microbiol Lett 278:48–55
Richard DJ, Sawers G, Sargent F, McWalter L, Boxer DH (1999) Transcriptional regulation in response to oxygen and nitrate of the operons encoding the [NiFe] hydrogenases 1 and 2 of Escherichia coli. Microbiology 145:2903–2912
Rossmann R, Sawers G, Bock A (1991) Mechanism of regulation of the formate-hydrogenlyase pathway by oxygen, nitrate and pH: definition of formate regulon. Mol Microbiol 5:2807–2814
Sasahara KC, Heinzinger NK, Barrett E (1997) Hydrogen sulfide production and fermentative gas production by Salmonella typhimurium require F0F1 ATPase activity. J Bacteriol 179:6736–6740
Sauter M, Bohm R, Bock A (1992) Mutational analysis of the operon (hyc) determining hydrogenase 3 formation in Escherichia coli. Mol Microbiol 6:1523–1532
Sawers RG, Ballantine SP, Boxer DH (1985) Differential expression of hydrogenase isoenzymes in Escherichia coli: evidence for a third isoenzyme. J Bacteriol 164:1324–1331
Soboh B, Pinske C, Kuhns M, Waclawek M, Ihling C, Trchounian K, Trchounian A, Sinz A, Sawers G (2011) The respiratory molybdo-selenoprotein formate dehydrogenases of Escherichia coli have hydrogen: benzyl viologen oxidoreductase activity. BMC Microbiol 11:173
Trchounian A (2004) Escherichia coli proton-translocating F0F1-ATP synthase and its association with solute secondary transpopters and/or enzymes of anaerobic oxidation-reduction under fermentation. Biochem Biophys Res Commun 315:1051–1057
Trchounian A, Bagramyan KA, Poladian A (1997) Formate hydrogen lyase are needed for proton-potassium exchange though the F0F1-ATPase and the TrkA system in anaerobically grown and glycolysing Escherichia coli. Curr Microbiol 35:201–206
Trchounian A, Kobayashi H (1998) Relationship of K+-uptaking system with H+-translocating ATPase in Enterococcus hirae, grown at high or low alkaline pH. Curr Microbiol 36:114–118
Trchounian K, Sanchez-Torres V, Wood TK, Trchounian A (2011) Escherichia coli hydrogenase activity and H2 production under glycerol fermentation at a low pH. Int J Hydrogen Energy 36:4323–4331
Trchounian K, Trchounian A (2009) Hydrogenase 2 is most and hydrogenase 1 is less responsible for H2 production by Escherichia coli under glycerol fermentation at neutral and slightly alkaline pH. Int J Hydrogen Energy 34:8839–8845
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trchounian, K., Pinske, C., Sawers, R.G. et al. Dependence on the F0F1-ATP synthase for the activities of the hydrogen-oxidizing hydrogenases 1 and 2 during glucose and glycerol fermentation at high and low pH in Escherichia coli . J Bioenerg Biomembr 43, 645–650 (2011). https://doi.org/10.1007/s10863-011-9397-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-011-9397-9