Abstract
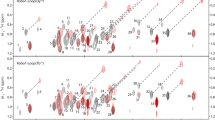
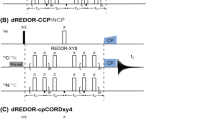
A simple NMR method is presented for the identification and assignment of phosphorylated serine and threonine residues in 13C- or 13C/15N-labeled proteins. By exploiting modest (~5 Hz) 2- and 3-bond 13C–31P scalar couplings, the aliphatic 1H–13C signals from phosphoserines and phosphothreonines can be detected selectively in a 31P spin-echo difference constant time 1H-13C HSQC spectrum. Inclusion of the same 31P spin-echo element within the 13C frequency editing period of an intraHNCA or HN(CO)CA experiment allows identification of the amide 1HN and 15N signals of residues (i) for which 13Cα(i) or 13Cα(i − 1), respectively, are coupled to a phosphate. Furthermore, 31P resonance assignments can be obtained by applying selective low power cw 31P decoupling during the spin-echo period. The approach is demonstrated using a PNT domain containing fragment of the transcription factor Ets-1, phosphorylated in vitro at Thr38 and Ser41 with the MAP kinase ERK2.




Similar content being viewed by others
Abbreviations
- CT:
-
Constant time
- CW:
-
Continuous wave
- DSS:
-
2,2-Dimethyl-2-silapentance-5-sulfonic acid
- ERK2:
-
Extracellular regulated kinase 2
- HSQC:
-
Heteronuclear single quantum correlation
- MAPK:
-
Mitogen activated protein kinase
- pSer:
-
Phosphoserine
- pThr:
-
Phosphothreonine
- TMP:
-
Trimethylphosphate
References
Bax A, Vuister GW, Grzesiek S, Delaglio F, Wang AC, Tschudin R, Zhu G (1994) Measurement of homo- and heteronuclear J couplings from quantitative J correlation. Methods Enzymol 239:79–105
Bienkiewicz EA, Lumb KJ (1999) Random-coil chemical shifts of phosphorylated amino acids. J Biomol NMR 15:203–206
Brauer M, Sykes BD (1984) Phosphorus-31 nuclear magnetic resonance studies of phosphorylated proteins. Methods Enzymol 107:36–81
Brutscher B (2002) Intraresidue HNCA and COHNCA experiments for protein backbone resonance assignment. J Magn Reson 156:155–159
Coligan JE, Dunn BM, Speicher DW, Wingfield PT (2008) Current protocols in protein science. Wiley Interscience, New York
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Flinders J, Dieckmann T (2006) NMR spectroscopy of ribonucleic acids. Prog NMR Spectrosc 48:137–159
Foulds CE, Nelson ML, Blaszczak AG, Graves BJ (2004) Ras/mitogen-activated protein kinase signaling activates Ets-1 and Ets-2 by CBP/p300 recruitment. Mol Cell Biol 24:10954–10964
Goddard TD, Kneeler DG (1999) Sparky 3, 3rd edn. University of California, San Francisco
Gschwind RM, Armbrüster M, Zubrzycki IZ (2004) NMR detection of intermolecular NH–OP hydrogen bonds between guanidinium protons and bisphosphonate moieties in an artificial arginine receptor. J Am Chem Soc 126:10228–10229
Hoffmann R, Reichert I, Wachs WO, Zeppezauer M, Kalbitzer HR (1994) 1H and 31P NMR spectroscopy of phosphorylated model peptides. Int J Pept Protein Res 44:192–198
Khokhlatchev A, Xu SC, English J, Wu PQ, Schaefer E, Cobb MH (1997) Reconstitution of mitogen-activated protein kinase phosphorylation cascades in bacteria—efficient synthesis of active protein kinases. J Biol Chem 272:11057–11062
Lescop E, Schanda P, Brutscher B (2007) A set of BEST triple-resonance experiments for time-optimized protein resonance assignment. J Magn Reson 187:163–169
Löhr F, Mayhew SG, Rüterjans H (2000) Detection of scalar couplings across NH…OP and OH…OP hydrogen bonds in a flavoprotein. J Am Chem Soc 122:9289–9295
Mackereth CD, Schärpf M, Gentile LN, MacIntosh SE, Slupsky CM, McIntosh LP (2004) Diversity in structure and function of the Ets family PNT domains. J Mol Biol 342:1249–1264
Markley JL, Bax A, Arata Y, Hilbers CW, Kaptein R, Sykes BD, Wright PE, Wüthrich K (1998) Recommendations for the presentation of NMR structures of proteins and nucleic acids—IUPAC-IUBMB-IUPAB inter-union task group on the standardization of data bases of protein and nucleic acid structures determined by NMR spectroscopy. Eur J Biochem 256:1–15
Mishima M, Hatanaka M, Yokoyama S, Ikegami T, Wälchli M, Ito Y, Shirakawa M (2000) Intermolecular 31P–15N and 31P–1H scalar coupling across hydrogen bonds formed between a protein and a nucleotide. J Am Chem Soc 122:5883–5884
Olsen JV, Bllagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M (2006) Global, in vivo, and site-specific phosphorylation dynamics in signalling networks. Cell 127:635–648
Pufall MA, Lee GM, Nelson ML, Kang H-S, Velyvis A, Kay LE, McIntosh LP, Graves BJ (2005) Variable control of Ets-1 DNA binding by multiple phosphates in an unstructured region. Science 309:142–145
Santoro J, King GC (1992) A constant-time 2D Overbodenhausen experiment for inverse correlation of isotopically enriched species. J Magn Reson 97:202–207
Sattler M, Schleucher J, Griesinger C (1999) Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog Nucl Magn Reson Specrosc 34:93–158
Schanda P, Kupce E, Brutscher B (2005) SOFAST–HMQC experiments for recording two-dimensional heteronuclear correlation spectra of proteins within a few seconds. J Biomol NMR 33:199–211
Schanda P, Van Melckebeke H, Brutscher B (2006) Speeding up three-dimensional protein NMR experiments to a few minutes. J Am Chem Soc 128:9042–9043
Seidel JJ, Graves BJ (2002) An ERK2 docking site in the pointed domain distinguishes a subset of ETS transcription factors. Genes Dev 16:127–137
Slupsky CM, Gentile LN, Donaldson LW, Mackereth CD, Seidel JJ, Graves BJ, McIntosh LP (1998) Structure of the Ets-1 pointed domain and mitogen-activated protein kinase phosphorylation site. Proc Natl Acad Sci USA 95:12129–12134
Smith JC, Figeys D (2008) Recent developments in mass spectrometry-based quantitative phosphoproteomics. Biochem Cell Biol 86:137–148
Suh JY, Tang C, Cai M, Clore GM (2005) Visualization of the phosphorylated active site loop of the cytoplasmic B domain of the mannitol transporter II (Mannitol) of the Escherichia coli phosphotransferase system by NMR spectroscopy and residual dipolar couplings. J Mol Biol 353:129–1135
Szyperski T, Fernandez C, Ono A, Wüthrich K, Kainosho M (1999) The 2D 31P spin-echo-difference constant-time [13C, 1H]-HMQC experiment for simultaneous determination of 3JH3′P and 3JC4′P in 13C-labeled nucleic acids and their protein complexes. J Magn Reson 140:491–494
Vogel HJ (1989) Phosphorus-31 nuclear magnetic resonance of phosphoproteins. Methods Enzymol 177:263–282
Vuister GW, Bax A (1992) Resolution enhancement and spectral editing of uniformly C-13-enriched proteins by homonuclear broad-band C-13 decoupling. J Magn Reson 98:428–435
Vuister G, Bax A (1993) Quantitative J correlation: a new approach for measuring homonuclear three-bond J(HN-HA) coupling constants in 15N-enriched proteins. J Am Chem Soc 115:7772–7777
Waas WF, Dalby KN (2002) Transient protein-protein interactions and a random-ordered kinetic mechanism for the phosphorylation of a transcription factor by extracellular-regulated protein kinase 2. J Biol Chem 277:12532–12540
Acknowledgements
We thank Robert Konrat and Adrien Favier for helpful discussions and technical help. L.P.M. gratefully acknowledges the sabbatical support of a Killam Faculty Research Fellowship, a Séjour Scientifique de Haut Niveau Award from the Scientific Services of the French Embassy in Canada, and a CIHR-CNRS International Exchange Grant. This research was supported by grants from National Cancer Institute of Canada with funds from the Canadian Cancer Society (to L.P.M.) and the National Institutes of Health grants GM38663 (to B.J.G.) and CA42014-I (to the Huntsman Cancer Institute for support of core facilities). B.J.G. also acknowledges funding from the Huntsman Cancer Institute/Huntsman Cancer Foundation. Instrument support was provided by the Canadian Institutes for Health Research (CIHR), the Canadian Foundation for Innovation (CFI), the British Columbia Knowledge Development Fund (BCKDF), the UBC Blusson Fund, and the Michael Smith Foundation for Health Research (MSFHR). B.B. acknowledges financial support from the Commissariat à l’Energie Atomique (CEA), the Centre National de la Recherche Scientifique (CNRS), the University Grenoble, and the French Research Agency (ANR).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
McIntosh, L.P., Kang, HS., Okon, M. et al. Detection and assignment of phosphoserine and phosphothreonine residues by 13C–31P spin-echo difference NMR spectroscopy. J Biomol NMR 43, 31–37 (2009). https://doi.org/10.1007/s10858-008-9287-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-008-9287-6