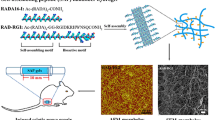
Abstract
Tissue engineering technology is applicable for study of nerve regeneration after spinal cord injury. Many natural and artificial scaffold are not applicable because of poor mechanical properties and cell compatibility. Polypeptides with fine three-dimensional structure and cell compatibility and are widely used in tissue engineering research. The purpose of this study was to verify the neuronal differentiation of neural stem cells by using self-polymerize dendritic polypeptide for spinal cord tissue engineering. Neural stem cells were isolated from cerebral cortex of neonatal SD rats.Conventional media was triggered the 1wt% nano peptide solution self polymerizated to formed a nano gel. The gel was tested by scanning electron microscope and transmission electron microscope. Neural stem cells were inoculated onto gel or on Polylysine-coated slides with fetal bovine serum or not. SD rats were randomized divided into four groups. neural stem cells and self-polymerized peptide were transplanted into spinal cord injury models. Then we test the Density of NF-positive axons in the spinal cord injury area at 8 weeks after surgery and MS score of the locomotive function of hind limbs among mice of four groups. Neural stem cells were showed anti Nestin (+), anti NSE (+), anti GFAP (+). The gel tested by scanning electron microscope was showed thick wall structure, another one tested by transmission electron microscope was showed self-polymerized dendritic nanofibers, which contains several spacings. The cells in serum group were differentiate into neurons, but non serum group were not. These results suggest that the self-assembling peptide nanofiber scaffold(SAPNS) were cytocompatible to neural stem cells which were differentiated into neurons. A large number of axonal regeneration and recovery of joint function of hind limb were appeared. The self-polymerized Peptide maybe used as practical tissue engineering materials as future.
Graphical abstract






Similar content being viewed by others
References
Brazda N, Voss C, Estrada V, Lodin H, Weinrich N, Seide K, Muller J, Muller HW. A mechanical microconnector system for restoration of tissue continuity and long-term drug application into the injured spinal cord. Biomaterials. 2013;34(38):10056–64. https://doi.org/10.1016/j.biomaterials.2013.09.057.
Oichi T, Oshima Y, Okazaki R, Azuma S. Preexisting severe cervical spinal cord compression is a significant risk factor for severe paralysis development in patients with traumatic cervical spinal cord injury without bone injury: a retrospective cohort study. Eur Spine J. 2016;25(1):96–102. https://doi.org/10.1007/s00586-015-4142-4.
Soubeyrand M, Laemmel E, Court C, Dubory A, Vicaut E, Duranteau J. Rat model of spinal cord injury preserving dura mater integrity and allowing measurements of cerebrospinal fluid pressure and spinal cord blood flow. Eur Spine J. 2013;22(8):1810–19. https://doi.org/10.1007/s00586-013-2744-2.
Zeng X, Qiu XC, Ma YH, Duan JJ, Chen YF, Gu HY, Wang JM, Ling EA, Wu JL, Wu W, Zeng YS. Integration of donor mesenchymal stem cell-derived neuron-like cells into host neural network after rat spinal cord transection. Biomaterials. 2015;53:184–01. https://doi.org/10.1016/j.biomaterials.2015.02.073.
Wu W, Lee SY, Wu X, Tyler JY, Wang H, Ouyang Z, Park K, Xu XM, Cheng JX. Neuroprotective ferulic acid (FA)-glycol chitosan (GC) nanoparticles for functional restoration of traumatically injured spinal cord. Biomaterials. 2014;35(7):2355–64. https://doi.org/10.1016/j.biomaterials.2013.11.074.
Weightman AP, Pickard MR, Yang Y, Chari DM. An in vitro spinal cord injury model to screen neuroregenerative materials. Biomaterials. 2014;35(12):3756–65. https://doi.org/10.1016/j.biomaterials.2014.01.022.
Wang Z, Nong J, Shultz RB, Zhang Z, Kim T, Tom VJ, Ponnappan RK, Zhong Y. Local delivery of minocycline from metal ion-assisted self-assembled complexes promotes neuroprotection and functional recovery after spinal cord injury. Biomaterials. 2017;112:62–71. https://doi.org/10.1016/j.biomaterials.2016.10.002.
Requejo-Aguilar R, Alastrue-Agudo A, Cases-Villar M, Lopez-Mocholi E, England R, Vicent MJ, Moreno-Manzano V. Combined polymer-curcumin conjugate and ependymal progenitor/stem cell treatment enhances spinal cord injury functional recovery. Biomaterials. 2017;113:18–30. https://doi.org/10.1016/j.biomaterials.2016.10.032.
Lai BQ, Wang JM, Duan JJ, Chen YF, Gu HY, Ling EA, Wu JL, Zeng YS. The integration of NSC-derived and host neural networks after rat spinal cord transection. Biomaterials. 2013;34(12):2888–01. https://doi.org/10.1016/j.biomaterials.2012.12.046.
Li X, Xiao Z, Han J, Chen L, Xiao H, Ma F, Hou X, Li X, Sun J, Ding W, Zhao Y, Chen B, Dai J. Promotion of neuronal differentiation of neural progenitor cells by using EGFR antibody functionalized collagen scaffolds for spinal cord injury repair. Biomaterials. 2013;34(21):5107–16. https://doi.org/10.1016/j.biomaterials.2013.03.062.
Gomes ED, Mendes SS, Leite-Almeida H, Gimble JM, Tam RY, Shoichet MS, Sousa N, Silva NA, Salgado AJ. Combination of a peptide-modified gellan gum hydrogel with cell therapy in a lumbar spinal cord injury animal model. Biomaterials. 2016;105:38–51. https://doi.org/10.1016/j.biomaterials.2016.07.019.
Gao M, Lu P, Bednark B, Lynam D, Conner JM, Sakamoto J, Tuszynski MH. Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials. 2013;34(5):1529–36. https://doi.org/10.1016/j.biomaterials.2012.10.070.
Lai BQ, Che MT, Du BL, Zeng X, Ma YH, Feng B, Qiu XC, Zhang K, Liu S, Shen HY, Wu JL, Ling EA, Zeng YS. Transplantation of tissue engineering neural network and formation of neuronal relay into the transected rat spinal cord. Biomaterials. 2016;109:40–54. https://doi.org/10.1016/j.biomaterials.2016.08.005.
Caron I, Rossi F, Papa S, Aloe R, Sculco M, Mauri E, Sacchetti A, Erba E, Panini N, Parazzi V, Barilani M, Forloni G, Perale G, Lazzari L, Veglianese P. A new three dimensional biomimetic hydrogel to deliver factors secreted by human mesenchymal stem cells in spinal cord injury. Biomaterials. 2016;75:135–47. https://doi.org/10.1016/j.biomaterials.2015.10.024.
Li G, Che MT, Zhang K, Qin LN, Zhang YT, Chen RQ, Rong LM, Liu S, Ding Y, Shen HY, Long SM, Wu JL, Ling EA, Zeng YS. Graft of the NT-3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials. 2016;83:233–48. https://doi.org/10.1016/j.biomaterials.2015.11.059.
Ohtake Y, Park D, Abdul-Muneer PM, Li H, Xu B, Sharma K, Smith GM, Selzer ME, Li S. The effect of systemic PTEN antagonist peptides on axon growth and functional recovery after spinal cord injury. Biomaterials. 2014;35(16):4610–26. https://doi.org/10.1016/j.biomaterials.2014.02.037.
Acknowledgements
We would like to thank Mr. Daiqiu Chen with Southern medical University for his helpful polishing of the manuscript, and Dr. Kai Huang with Tongde hospital of Zhejiang province for his technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This study was approved by institutional ethics committee of southern medical university in China, followed international guidelines for using animals in study. (Approval No. 2017-0128).
Additional information
Jun ming Wan and Liang le Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wan, J.m., Liu, L.l., Zhang, J.f. et al. Promotion of neuronal regeneration by using self-polymerized dendritic polypeptide scaffold for spinal cord tissue engineering. J Mater Sci: Mater Med 29, 6 (2018). https://doi.org/10.1007/s10856-017-6010-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-6010-8