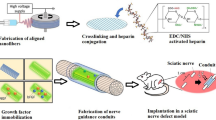
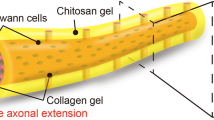
Abstract
Nerve guidance conduit (NGC) is a potential alternative to autologous nerve for peripheral nerve regeneration. A promising therapeutic strategy is to modify the nerve guidance conduit intraluminal microenvironment using physical and/or chemical guidance cues. In this study, a neurotrophic peptide-functionalized self-assembling peptide nanofiber hydrogel that could promote PC12 cell adhesion, proliferation, and neuronal differentiation in vitro was prefilled in the lumen of a hollow chitosan tube (hCST) to accelerate axonal regeneration in a rat sciatic nerve defect model. The functionalized self-assembling peptide was developed by introducing a neurotrophic peptide (RGI, RGIDKRHWNSQ) derived from brain-derived neurotrophic factor (BDNF) to the C-terminus of the self-assembling peptide RADA16-I (Ac-(RADA)4-CONH2). Morphological, histological, electrophysiological, and functional analyses demonstrated that the RGI-functionalized, self-assembling, peptide nanofiber hydrogel RAD/RGI could produce a neurotrophic microenvironment that markedly improved axonal regeneration with enhanced re-myelination and motor functional recovery.

Similar content being viewed by others
References
Burnett, M. G.; Zager, E. L. Pathophysiology of peripheral nerve injury: A brief review. Neurosurg. Focus 2004, 16, 1–7.
Pfister, B. J.; Gordon, T.; Loverde, J. R.; Kochar, A. S.; Mackinnon, S. E.; Cullen, D. K. Biomedical engineering strategies for peripheral nerve repair: Surgical applications, state of the art, and future challenges. Crit. Rev. Biomed. Eng. 2011, 39, 81–124.
Ray, W. Z.; Mackinnon, S. E. Management of nerve gaps: Autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp. Neurol. 2010, 223, 77–85.
Gu, X. S.; Ding, F.; Williams, D. F. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 2014, 35, 6143–6156.
Faroni, A.; Mobasseri, S. A.; Kingham, P. J.; Reid, A. J. Peripheral nerve regeneration: Experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 2015, 82–83, 160–167.
Kehoe, S.; Zhang, X. F.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572.
Toba, T.; Nakamura, T.; Shimizu, Y.; Matsumoto, K.; Ohnishi, K.; Fukuda, S.; Yoshitani, M.; Ueda, H.; Hori, Y.; Endo, K. Regeneration of canine peroneal nerve with the use of a polyglycolic acid-collagen tube filled with lamininsoaked collagen sponge: A comparative study of collagen sponge and collagen fibers as filling materials for nerve conduits. J. Biomed. Mater. Res. 2001, 58, 622–630.
Cao, J. N.; Sun, C. K.; Zhao, H.; Xiao, Z. F.; Chen, B.; Gao, J.; Zheng, T. Z.; Wu, W.; Wu, S.; Wang, J. Y. et al. The use of laminin modified linear ordered collagen scaffolds loaded with laminin-binding ciliary neurotrophic factor for sciatic nerve regeneration in rats. Biomaterials 2011, 32, 3939–3948.
Kim, Y. P.; Lee, G. S.; Kim, J. W.; Kim, M. S.; Ahn, H. S.; Lim, J. Y.; Kim, H. W.; Son, Y. J.; Knowles, J. C.; Hyun, J. K. Phosphate glass fibres promote neurite outgrowth and early regeneration in a peripheral nerve injury model. J. Tissue Eng. Regen. Med. 2015, 9, 236–246.
Carriel, V.; Garrido-Gómez, J.; Hernández-Cortés, P.; Garzón, I.; García-García, S.; Sáez-Moreno, J. A.; del Carmen Sánchez-Quevedo, M.; Campos, A.; Alaminos, M. Combination of fibrin-agarose hydrogels and adipose-derived mesenchymal stem cells for peripheral nerve regeneration. J. Neural Eng. 2013, 10, 026022.
Ohta, M.; Suzuki, Y.; Chou, H.; Ishikawa, N.; Suzuki, S.; Tanihara, M.; Suzuki, Y.; Mizushima, Y.; Dezawa, M.; Ide, C. Novel heparin/alginate gel combined with basic fibroblast growth factor promotes nerve regeneration in rat sciatic nerve. J. Biomed. Mater. Res. A 2004, 71A, 661–668.
Du, J. R.; Liu, J. H.; Yao, S. L.; Mao, H. Q.; Peng, J.; Sun, X.; Cao, Z.; Yang, Y. D.; Xiao, B.; Wang, Y. G. et al. Prompt peripheral nerve regeneration induced by a hierarchically aligned fibrin nanofiber hydrogel. Acta Biomater. 2017, 55, 296–309.
Liu, X.; Pi, B.; Wang, H.; Wang, X. M. Self-assembling peptide nanofiber hydrogels for central nervous system regeneration. Front. Mater. Sci. 2015, 9, 1–13.
Hauser, C. A. E.; Zhang, S. G. Designer self-assembling peptide nanofiber biological materials. Chem. Soc. Rev. 2010, 39, 2780–2790.
Yang, Y. L.; Khoe, U.; Wang, X. M.; Horii, A.; Yokoi, H.; Zhang, S. G. Designer self-assembling peptide nanomaterials. Nano Today 2009, 4, 193–210.
Zhan, X. D.; Gao, M. Y.; Jiang, Y. W.; Zhang, W. W.; Wong, W. M.; Yuan, Q. J.; Su, H. X.; Kang, X. N.; Dai, X.; Zhang, W. Y. et al. Nanofiber scaffolds facilitate functional regeneration of peripheral nerve injury. Nanomedicine 2013, 9, 305–315.
Sun, Y. Q.; Li, W.; Wu, X. L.; Zhang, N.; Zhang, Y. N.; Ouyang, S. Y.; Song, X. Y.; Fang, X. Y.; Seeram, R.; Xue, W. et al. Functional self-assembling peptide nanofiber hydrogels designed for nerve degeneration. ACS Appl. Mater. Interfaces 2016, 8, 2348–2359.
Fobian, K.; Owczarek, S.; Budtz, C.; Bock, E.; Berezin, V.; Pedersen, M. V. Peptides derived from the solvent-exposed loops 3 and 4 of BDNF bind TrkB and p75(NTR) receptors and stimulate neurite outgrowth and survival. J. Neurosci. Res. 2010, 88, 1170–1181.
Koliatsos, V. E.; Clatterbuck, R. E.; Winslow, J. W.; Cayouette, M. H.; Prices, D. L. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron 1993, 10, 359–367.
Tolwani, R. J.; Cosgaya, J. M.; Varma, S.; Jacob, R.; Kuo, L. E.; Shooter, E. M. BDNF overexpression produces a long-term increase in myelin formation in the peripheral nervous system. J. Neurosci. Res. 2004, 77, 662–669.
Gordon, T. The role of neurotrophic factors in nerve regeneration. Neurosurg. Focus 2009, 26, E3.
Chau, Y.; Luo, Y.; Cheung, A. C. Y.; Nagai, Y.; Zhang, S. G.; Kobler, J. B.; Zeitels, S. M.; Langer, R. Incorporation of a matrix metalloproteinase-sensitive substrate into selfassembling peptides—A model for biofunctional scaffolds. Biomaterials 2008, 29, 1713–1719.
Böhm, G.; Muhr, R.; Jaenicke, R. Quantitative analysis of protein far UV circular dichroism spectra by neural networks. Protein. Eng. 1992, 5, 191–195.
Kloxin, A. M.; Benton, J. A.; Anseth, K. S. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials 2010, 31, 1–8.
Bain, J. R.; Mackinnon, S. E.; Hunter, D. A. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast. Reconstr. Surg. 1989, 83, 129–136.
Wang, X. M.; Horii, A.; Zhang, S. G. Designer functionalized self-assembling peptide nanofiber scaffolds for growth, migration, and tubulogenesis of human umbilical vein endothelial cells. Soft Matter 2008, 4, 2388–2395.
Yokoi, H.; Kinoshita, T.; Zhang, S. G. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc. Natl. Acad. Sci. USA 2005, 102, 8414–8419.
Hong, Y.; Legge, R. L.; Zhang, S.; Chen, P. Effect of amino acid sequence and pH on nanofiber formation of self-assembling peptides EAK16-II and EAK16-IV. Biomacromolecules 2003, 4, 1433–1442.
Zhang, S. G. Designer self-assembling peptide nanofiber scaffolds for study of 3-D cell biology and beyond. Adv. Cancer Res. 2008, 99, 335–362.
Horii, A.; Wang, X. M.; Gelain, F.; Zhang, S. G. Biological designer self-assembling peptide nanofiber scaffolds significantly enhance osteoblast proliferation, differentiation and 3-D migration. PLoS One 2007, 2, e190.
Liu, X.; Wang, X. M.; Wang, X. J.; Ren, H.; He, J.; Qiao, L.; Cui, F. Z. Functionalized self-assembling peptide nanofiber hydrogels mimic stem cell niche to control human adipose stem cell behavior in vitro. Acta Biomater. 2013, 9, 6798–6805.
Yao, S. L.; Liu, X.; Yu, S. K.; Wang, X. M.; Zhang, S. M.; Wu, Q.; Sun, X. D.; Mao, H. Q. Co-effects of matrix low elasticity and aligned topography on stem cell neurogenic differentiation and rapid neurite outgrowth. Nanoscale 2016, 8, 10252–10265.
Flanagan, L. A.; Ju, Y. E.; Marg, B.; Osterfield, M.; Janmey, P. A. Neurite branching on deformable substrates. NeuroReport 2002, 13, 2411–2415.
Ozawa, H.; Matsumoto, T.; Ohashi, T.; Sato, M.; Kokubun, S. Comparison of spinal cord gray matter and white matter softness: Measurement by pipette aspiration method. J. Neurosurg. 2001, 95, 221–224.
Wilhelm, J. C.; Xu, M.; Cucoranu, D.; Chmielewski, S.; Holmes, T.; Lau, K.; Bassell, G. J.; English, A. W. Cooperative roles of BDNF expression in neurons and schwann cells are modulated by exercise to facilitate nerve regeneration. J. Neurosci. 2012, 32, 5002–5009.
Costa, L. M.; Simões, M. J.; Maurício, A. C.; Varejão, A. S. P. Methods and protocols in peripheral nerve regeneration experimental research: Part IV-kinematic gait analysis to quantify peripheral nerve regeneration in the rat. Int. Rev. Neurobiol. 2009, 87, 127–139.
Stang, F.; Fansa, H.; Wolf, G.; Reppin, M.; Keilhoff, G. Structural parameters of collagen nerve grafts influence peripheral nerve regeneration. Biomaterials 2005, 26, 3083–3091.
Pace, L. A.; Plate, J. F.; Smith, T. L.; Van Dyke, M. E. The effect of human hair keratin hydrogel on early cellular response to sciatic nerve injury in a rat model. Biomaterials 2013, 34, 5907–5914.
Shi, W.; Huang, C. J.; Xu, X. D.; Jin, G. H.; Huang, R. Q.; Huang, J. F.; Chen, Y. N.; Ju, S. Q.; Wang, Y.; Shi, Y. W. et al. Transplantation of RADA16-BDNF peptide scaffold with human umbilical cord mesenchymal stem cells forced with CXCR4 and activated astrocytes for repair of traumatic brain injury. Acta Biomater. 2016, 45, 247–261.
Frostick, S. P.; Yin, Q.; Kemp, G. J. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery 1998, 18, 397–405.
Johnson, E. O.; Zoubos, A. B.; Soucacos, P. N. Regeneration and repair of peripheral nerves. Injury 2005, 36, S24–S29.
Colen, K. L.; Choi, M.; Chiu, D. T. W. Nerve grafts and conduits. Plast. Reconstr. Surg. 2009, 124, e386–e394.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Nos. 31771056 and 31771052), the Tsinghua University Initiative Scientific Research Program (No. 20161080091) and the 111 Project (No. B17026). We would like to thank Dr. Yueteng Wei at Bruker (Beijing, China) for help in AFM. We thank to Xiaodan Mu at Harbin Medical University and Yifan Liu at Tsinghua University for the helpful suggestion of experiments.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
12274_2018_2041_MOESM1_ESM.pdf
A neurotrophic peptide-functionalized self-assembling peptide nanofiber hydrogel enhances rat sciatic nerve regeneration
Rights and permissions
About this article
Cite this article
Lu, J., Sun, X., Yin, H. et al. A neurotrophic peptide-functionalized self-assembling peptide nanofiber hydrogel enhances rat sciatic nerve regeneration. Nano Res. 11, 4599–4613 (2018). https://doi.org/10.1007/s12274-018-2041-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-018-2041-9