Abstract
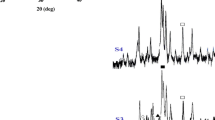
Solution combustion technique has been used to prepare hydroxypatite (HAp) powder from calcium nitrate, di-ammonium hydrogen phosphate and citric acid precursors. Phase evolution has been studied as a function of calcination temperatures. The crystal structure, phase purity and stiochiometry of phase have been studied by Rietveld analysis of the calcined powder. It was observed that the prepared Hap powder was phase pure and stoichiometric. The sintering behaviour and sintering kinetics of the HAp compact has been studied by dilatometer. Activation energy for sintering has been calculated from the dilatometer results. Grain boundary diffusion was found to be the dominant densification mechanism during the initial stage of sintering. The activation energy for sintering (438 kJ/mol) was found to be in excellent agreement with reported value.
Similar content being viewed by others
References
L.L. HENCH, J. Am. Ceram. Soc. 74 (1991) 1487.
H. AOKI, in “Science and medical applications of hydroxyapatite” (Japanese Association of Apatite Science, Tokyo, Japan, 1991).
W. SUCHANEK, M. YOSHIMURA, J. Mater. Res. 13 (1998) 94.
K. YAMASHITA, T. KANAZAWA, in “Inorganic phosphate materials” Kanazawa T, (Ed.) (Kodansha & Elsevier, Tokyo and Amsterdam, 1989) p. 15.
R. Z. LEGEROS, in “Calcium phosphates in oral biology and medicine” (Karger, Basel, Switzerland) 1991.
L. BERNARD, M, FRECHE, J. L. LACOUT and B. BISCANS, Powder Technol. 103 (1999) 19.
LIU, T. S. CHIN, L. S. LAI, S. Y. CHIU, K. H. CHUNG, C. S. CHANG and M. T. LIU, Ceram. Int. 23 (1997) 19.
D-M. LIU, T. TROCZYNSKI and W. J. T. SENG, Biomaterials 22 (2001) 1721.
D.-M. LIU, Q. YANG, T. TROCZYNSKI and W. J. TSENG, Biomaterials 23 (2002) 1679.
G. K. LIM, J. WANG, S. C. NG, C. H. CHEW and L. M. GAN, Biomaterials 18 (1997) 1433.
K. C. B. YEONG, J. WANG and S. C. NG, Biomaterials 22 (2001) 2705.
W. L. SUCHANEK, P. SHUK, K. BYRAPPA, R. E. RIMAN, K. S. TENHUISEN and V. F. JANAS, Biomaterials 23 (2002) 699.
D. A. FUMO, M. R. MORELLI and A. M. SEGADAES, Mater. Res. Bull 31 (1996) 1243.
T. YE, Z. GUIWEN, Mater. Res. Bull. 32 (1997) 501.
D. A. FUMO, J. R. JURADO, A. M. SEGADAES and J. R. FRADE, Mater. Res. Bull. 32 (1997) 1459.
R. E. JUóREZ, D. G. LAMAS, G. E. LASCALEA, D. E. WALSOE and N. E. RECA, J. Eur. Ceram. Soc. 20 (2000) 133.
H. TAGUCHI, S. YAMADA, M. NAGAO, Y. ICHIKAWA, K. TABATA, Mater. Res. Bull. 37 (2002) 69.
R. K. SELVAN, C. O. SUGUSTIN, L. J. BERCHMANS and R. SARASWATHI, Mater. Res. Bull. 38 (2003) 41.
J. HUANG, H. ZHUANG, W. L. LI, Mater. Res. Bull. 38 (2003) 149.
M. MUTHURAMAN, K. C. PATIL, Mater. Res. Bull. 33 (1998) 655.
K. ADHIKARY, M. TAKAHASHI and SH. KIKKAWA, Mater. Res. Bull. 33 (1998) 1845.
R. SUKUMAR, W. LIWU, S. WOLFGANG and A. FRITZ, Mater. Lett. 39 (1999) 138.
H. K. VARMA, S. N. KALKURA and R. SIVAKUMAR, Ceram. Int. 24 (1998) 467.
A. CUNEYT TAS, J. Eur. Ceram. Soc. 20 (2000) 2389.
Y. HAN, S. LI, X. WANG and X. CHEN, Mater. Res. Bull. 39 (2004) 25.
MAUD stands for Material Analysis Using Diffraction. It is based on the RITA/RISTA method by H.-R. WENK, S. MATTHIES and L. LUTTEROTTI, BERKELEY, University of Claifornia, Version 1.999 (3rd December 2003).
H. KLUG, L. E. ALEXANDER, in “X-ray diffraction procedures for polycrystalline and amorphous materials” (John Wiley and Sons, New York, 1974) p. 618–708.
K. SUDARSANAN and R. A. YOUNG, Acta. Crystallogr. B25 (1969) 1534.
R. M. WILSON and J. C. ELLIOTT AND S.E.P. DOWKER, Am. Mineral. 84 (1999) 1406.
G. R. FISCHER, P. BARDHAN and J. E. GEIGER, J. Mater. Sci. Lett. 2 (1983) 577.
S. NAKAMURA, R. OTSUKA, H. OAKI, M. AKAO, N. MIURA and T AMAMOTO, Thermochim. Acta. 165 (1990) 57.
S. RAYNAUD, E. CHAMPION and D. BERNACHE-ASSOLLANT, Biomaterials 23 (2002) 1073.
H. Y. JUANG and M. H. HON, Biomaterials 17 (1996) 2059.
F. TIETZ, F. J. DIAS, D. SIMWONIS and D. STOVER, J. Euro. Ceram. Soc. 20 (2000) 1023.
W. S. YOUNG and I. B. CUTLER, J. Am. Ceram. Soc. 53 (1970) 659.
V. A. IVENSEN, in “Densification of metal powders during sintering” Consultants Bureau, New York, 1973.
S. BAILLIEZ and A. NZIHOU, Chem. Eng. J. 98 (2004) 141.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pratihar, S.K., Garg, M., Mehra, S. et al. Phase evolution and sintering kinetics of hydroxyapatite synthesized by solution combustion technique. J Mater Sci: Mater Med 17, 501–507 (2006). https://doi.org/10.1007/s10856-006-8932-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10856-006-8932-4