Abstract
In this paper, the sintering behavior of the SrB2Si2O8 ceramics based on the SrO–B2O3–SiO2 composition has been investigated. The influence of the BaO addition to the SrO–B2O3–SiO2 ceramics on the sintering behavior has been also examined. It shows that, due to the low-meting characteristics of the SrO–B2O3 binary composition, the SrB2Si2O8 ceramics with peculiar dielectric properties can easily prepared by the introduction of SiO2 to the SrO–B2O3 binary compositions at a sintering temperature of 950 °C. Additionally, the introduction of BaO to SrO–B2O3–SiO2 ceramics has an obvious effect on the sintering behavior of the SrB2Si2O8 ceramics. Also, the dielectric properties of the SrB2Si2O8 ceramics are correlative to the phase compositions and the microstructures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, low-temperature co-fired ceramics (LTCC) technologies have been attracting much attention because of the rapid growth of the wireless communication systems and microwave products in the consumer electronic market [1]. The low-temperature sintering technology is an important way to enable its advantageous utilization for today’s packaging process in microelectronics and in microwave modules. Particularly, because the LTCC materials can be sintered at a temperature of below 1,000 °C, the embedded components and transmission lines can be made by using the highly conductive metals, e.g. silver, copper, gold, with low conductor loss and low electrical resistance at high frequencies [1, 2]. So far, glass–ceramics and ceramic/glass composites have been widely researched for LTCC application due to their tailored physical properties and low sintering temperature [3]. The preparation of glass–ceramics is achieved by a devitrification of glass materials. Also, the ceramic/glass composites are usually fabricated by using glasses as a matrix and functional ceramic particles as filler, which is convenient to the property design of the composite materials. Because a liquid-phase sintering can supply various facilities for the achievement of the densification sintering [4], glass materials play an important role in the fabrication of LTCC materials. Nevertheless, the manufacture of most glass materials is usually a complex process including high-temperature melting, rapid cooling and crystallization. Its energy consumption is relatively high. Consequently, it should be considered by choosing more convenient methods to improve the preparation process to achieve an energy-saving LTCC preparation process. At present, there are some multiple oxide compositions with a melting temperature below 1,000 °C [5–10], such as CaO–B2O3–SiO2, BaO–ZnO–SiO2–B2O3, BaO–B2O3–SiO2, Li2O–MgO–P2O5, ZnO–TeO2 etc., which have the potential to develop single crystal phase ceramics, e.g., CaSiO3, Zn2SiO4, BaSiO3, LiMgPO4 and Zn2Te3O8, etc., with peculiar physical properties and a low sintering temperature. The low-melting compositions can supply sufficient amounts of liquid phase for the crystal phase formations and the achievement of the densification sintering at relative low sintering temperature. In turn, dielectric and thermal properties of the low-temperature ceramics can be tailored in terms of these crystal phases.
In this work, a SrB2Si2O8 ceramics with a low sintering temperature is researched as LTCC candidate materials, the SrB2Si2O8 ceramics based on SrO–B2O3–SiO2 composition are fabricated by the introduction of a SiO2 component to a SrO–B2O3 binary composition with low-melting characteristics. Presently, few researches have reported the luminescent properties of the SrB2Si2O8 crystals used as a luminescence matrix [11, 12], but a very little research concerned on the sintering behaviors and the electric properties of the SrB2Si2O8 ceramics. The objective of the work is to discuss the sintering behavior of the SrO–B2O3–SiO2 ceramics and its dielectric properties so that it can be developed for the possible application in the LTCC technologies.
2 Experimental
H3BO3, SrCO3, H2SiO3 and Ba(OH)2·8H2O with purity higher than 99 % were used as the starting materials. According to the designed compositions listed in Table 1, the starting materials were weighed and milled with de-ionized water plus little ammonia for 7 h. The weight ratio of the water to solid was regulated to 2/1. Upon treatment, the slurries were dried at 80 °C. Subsequently, the powders with the particle size of 0.5–1.0 µm were molded into green bodies under a compressive stress of 25 MPa. Finally, the SrB2Si2O8 ceramics specimens were obtained by continuously sintering the green bodies at 600 °C for 3 h and 950 °C for 2 h. The microstructures of the specimens were analyzed by using a color 3D laser microscope (KEYENCE, VK-9700, Japan), a X-ray powder diffraction patterns and a differential scanning calorimetry (NETZSCH STA449C, Germany, heating at a rate of 10 °C min−1), the X-ray powder diffraction patterns were recorded on a D/Max-IIIA machine (Rigaku Industrial Corporation, Japan) using Cu Kα Radiation (40 kV, 30 mA) with a scanning rate of 2° min−1. Dielectric properties and quality value Q × f at microwave frequencies were measured with a network analyzer (Model Agilent E363A, USA) by using Hakki-Coleman dielectric resonator method.
3 Results and discussion
Sintering, especially a liquid-phase sintering is crucial to achieve a densification microstructure for LTCC materials. In addition, the densification sintering of ceramic materials also depend on sintering temperature, time as well as chemical and physical characteristics of starting materials such as particle size, chemical compositions, etc. Especially, the achievement of a low-temperature sintering for ceramics more mainly depends on the low-melting components existed in the ceramics materials. As well known from the phase diagram of a SrO–B2O3 binary system [13], the liquid phase of the SrO–B2O3 composition can be formed at least 950 °C whereas the low-temperature liquid phase zone is corresponded to the SrO–B2O3 composition with a 60–80 mol% B2O3 content, which consequently guarantee the low-temperature preparation of the SrO–B2O3 melts. However, the SrO–B2O3 melts can not completely contribute crucial advantages for physical properties such as thermal and dielectric properties. Therefore, the introduction of other oxides, e.g., SiO2, to the SrO–B2O3 compositions is expected to obtain the functional mineral phase and consequently achieve the fabrication of the low-temperature sintering ceramics with peculiar physical properties. Especially, the preparation process of the SrO–B2O3–SiO2 ceramics is based on an aqueous suspension route, and by the means of a chemical combination, hydrated borate compounds with a low-melting temperature and precursor compounds of the functional mineral phases can be formed from the suspension solutions of H3BO3, H2SiO3 and SrCO3, which consequently can provide more possibilities for the fabrication of the low temperature sintering ceramics with peculiar physical properties.
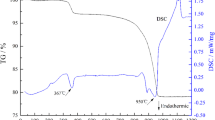
As shown in Fig. 1, the specimen SB with a SrO·3B2O3 composition have been basically transformed into a glassy form by a sintering processing for 2 h at 950 °C, indicating that a feasible SrO–B2O3 binary composition possess a low-melting characteristics. In comparison with the specimen SB, the specimen SB-1 with a SrO·2B2O3 composition also shows a melting form but it is not be transformed completely into a glassy form. It means that the B2O3 content have an obvious effect on the melting characteristics of the SrO–B2O3 composition, the result is in agreement with the SrO–B2O3 binary phase diagram. On the other hand, the introduction of SiO2 to the SrO–B2O3 composition do not obviously change the melting characteristics of the SrO–B2O3 composition but the sintering behavior of the SrO–B2O3–SiO2 melts seems to be changed by a feasible increase of the SiO2 content. As shown in Fig. 2, it is estimated that strontium borate compounds (crystallization peak, A) have been formed at about 700 °C and subsequently the compound crystals are gradually melted due to a elevating temperature and finally transformed into a glassy form. The melting characteristics depending on temperature for the specimen SB is similar to that for the specimen SB-3. It is speculated that the melting characteristics of the two specimens should be correlative to the strontium borate compounds existed in the slurries. As a result, it means that the B2O3 content can be availably decreased as the increase of the SiO2 content in the SrO–B2O3–SiO2 compositions and consequently achieve the low-temperature preparation of the SrO–B2O3–SiO2 ceramics. In addition, as shown in DTA cure of the specimen SB-3, it is also found that, besides the peak A at about 700 °C, another crystallization peak (marker B) are appeared at about 930 °C. It indicates that the rational introduction of SiO2 to the SrO–B2O3 composition can cause a formation of new crystal phases and more possibly obtain the low-temperature sintering SrO–B2O3–SiO2 ceramics. According to the results, several SrO–B2O3–SiO2 ceramic specimens have been prepared by a sintering process at 950 °C and the XRD patterns are represented in Fig. 3.
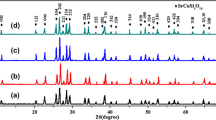
As shown in Fig. 3, the major mineral phase of the SrO–B2O3–SiO2 ceramic specimens is a SrB2Si2O8 crystal phase and SiO2 crystal phases are also appeared in the XRD pattern of the specimen SBS-6. It indicates that the SrB2Si2O8 crystal phase can be formed easily from the SrO–B2O3–SiO2 ceramic specimens via the present process. The formation of SiO2 crystals should be owing to the crystallization of the SiO2 residual polymer molecules during sintering, since the specimen SBS-6 has a relatively high SiO2 content. In fact, the formation of the mineral phases in the SrO–B2O3–SiO2 ceramics and its sintering behavior are more mainly decided by the compounds existed in its slurries. Figure 4 represents the XRD patterns of the SrO–B2O3–SiO2 ceramic slurries. It shows that the SrO–B2O3–SiO2 ceramic slurries mainly contain strontium borate hydrates and a small quantity of residual starting materials or polymer molecules. It is estimated that the strontium borate hydrates with a low-melting characteristics can supply a liquid-phase sintering aid for the achievement of the densification microstructures of the SrO–B2O3–SiO2 ceramics during sintering, and at the same time cause the available dissolution of the SiO2 polymer molecules to a SrO–B2O3 liquid phase to form the SrB2Si2O8 crystal phase. So the existence of the strontium borate hydrates in the slurries is crucial to the fabrication of the SrB2Si2O8 ceramics. In the case of the microstructures, Fig. 5 gives the photomicrographs of the SrB2Si2O8 ceramics; it indicates that, after be sintered at 950 °C, the SrB2Si2O8 ceramics possess the dense microstructures besides the specimens SBS-1 and SBS-5, whose microstructures are porous. The formation of the porous microstructures is usually attributed to the absence of a liquid phase in ceramic materials, since the pores can not be filled completely with an enough liquid phase during sintering. In addition, an over sintering can also results in the porous microstructures due to an occurrence of an excessive amounts of liquid phases during sintering. Therefore, the microstructures of the specimens SBS-1 and SBS-5 should be more possibly correlative to their chemical compositions such as B2O3 or SiO2 content, since the high B2O3 content can increase amounts of a liquid phase but the high SiO2 can decrease it as a result of a crystallization of SiO2 polymer molecules. In addition, the microstructures and the densities are also further influenced by some correlative factors such as crystallization and an existence of residual glassy phases. The further research needs a more detailed investigation on the relationship between the chemical composition and the amounts of a liquid phase in the future works.
On the other hand, the sintering behavior of the SrO–B2O3–SiO2 ceramics can be also influenced by the introduction of BaO to the SrO–B2O3–SiO2 composition. As known from the phase diagram of the binary system BaO–B2O3 [14], the onset of a liquid phase in the binary system BaO–B2O3 can be observed at least 869 °C, it means that the eutectic temperature of the binary system BaO–B2O3 is lower than that of the binary system SrO–B2O3. So the introduction of BaO to the SrO–B2O3–SiO2 ceramics is expected to improve the sintering behavior so as to cause a good densification sintering for the SrB2Si2O8 ceramics. Figure 6 gives the XRD patterns of the specimens SBSB-1 and SBSB-2, which are prepared by a replace of 5 wt% BaO for the SrO in the specimens SBS-5 and SBS-6, respectively, and a sintering process for 2 h at 950 °C. As shown in Fig. 6, the major mineral phases of the specimens SBSB-1 and SBSB-2 are a SrB2Si2O8 crystal phase and little other mineral phases, e.g., SiO2 crystal phases. It indicates that the feasible replace of BaO for the SrO in the SrO–B2O3–SiO2 ceramics can not obviously change the phase composition of the SrB2Si2O8 ceramics. But the effect of the BaO introduction on the microstructures is greatly distinct. As shown in Fig. 7, the photomicrograph of the specimen SBSB-1 shows a good crystallization of the SrB2Si2O8 crystals due to the existence of a sufficient liquid phase, whereas the photomicrographs of the specimen SBSB-2 also exhibit weak microstructures as a result of the absence of a liquid phase that are possibly attributed to a high SiO2 content in the composition. As shown In Fig. 8, it is found that, besides SrCO3 compound and SiO2 polymer molecules, the slurries of the specimens SBSB-1 and SBSB-2 contain only little barium borates hydrates. The appearance of the barium borates hydrates in the slurries of the SrO–B2O3–SiO2 ceramics should be relative to the complicated exchange of Ba and Sr ions in the aqueous suspension. It implies that the chemical-bond preference of the B2O3 polymer molecules is more tending to Ba ion rather than Sr ion, thus it can hinder the formation of strontium borate hydrates in the slurries of the specimens SBSB-1 and SBSB-2. Furthermore, as the compounds with low-melting characteristics [8], the appearance of the barium borates hydrates in the SrO–B2O3–SiO2 ceramics can further cause more liquid phase during sintering and at a certain extent facilitate the densification sintering of the SrO–B2O3–SiO2 ceramics. But as presented in Figs. 9, 10, with the BaO replace for the SrO in the SrB2Si2O8 ceramics, it does not only change the mineral phase composition of the SrO–B2O3–SiO2 ceramics but also result in a weak sintering behavior such as an over sintering phenomenon. In Fig. 10, the picture of the BaO–SrO–B2O3–SiO2 ceramics specimens SBSB-3, 4, 5, 6 have obviously shown that an over-sintering phenomenon have been shown on the pictures of these specimens with high BaO and B2O3 content. Moreover, the specimens SBSB-3, 4, 5, 6 sintered at 850 and 900 °C, respectively looks totally like similar with the specimens sintered at 950 °C. It indicates that the high BaO–B2O3 components in the ceramics can hinder the achievement of a densification sintering due to an over sintering. It is estimated that the occurrence of the over-sintering phenomenon should be attributed to the existence of the excessive liquid phases in the ceramics. Similarly, it also more easily causes a crystallization of the SiO2 polymer molecules and finally forms SiO2 crystals. Consequently, the weak sintering behavior is unbeneficial to achieve a good microstructures and dielectric properties. The regulation of the BaO and B2O3 content in the composition of the SrB2Si2O8 ceramics is equally important to achieve a single SrB2Si2O8 crystal phase and a good sintering behavior.
Generally, our investigations have indicated that the SrO–B2O3 composition with low-melting characteristics can play an important role in the fabrication of the SrB2Si2O8 ceramics. The SrO–B2O3 composition, as a liquid phase, in the ceramics can availably drive the various particles and polymer molecular dispersing and combining with each other to form the mineral phases with thermodynamic stability during sintering. On the basis of the classical theory of the liquid phase sintering [15], the sintering behavior of the low-temperature sintering ceramics can be understood as the mechanism that, by a liquid phase flow, the phase formation and the occurrence of the densification were achieved along with crystal particle dispersing, rearrangement, solution-precipitation and solid-stat sintering.
As far as the dielectric properties of the SrB2Si2O8 ceramics is concerned, the Q × f value and the dielectric constant and loss of the specimens are listed in Table 2. It shows that the dielectric constants of the SrB2Si2O8 ceramics have the values of about 4 and the dielectric losses are in a range of 10−3–10−4. Because of the porous microstructures, the dielectric properties of the specimens SBS-1, SBS-5 and SBSB-2 are relatively weaker than that of other specimens. In addition, an amount of the residual glassy phases in the specimen SBSB-1, which originated from the excessive liquid phases existed in the ceramics during sintering, should be responsible for its relative low dielectric constant. Also, it is found that the specimen SBS-4, whose chemical composition contains a reasonable B2O3 content and is closed the composition of a nature mineral, pekovite [16], obtain a good sintering behavior and a high Q × f value. As well known, SrB2Si2O8 is an alkaline borosilicate mineral with a Pekovite (Danburite) crystal structure. At present, most of alkaline borosilicate minerals have been widely researched for the applications in the immobilization on high-level radioactive wastes and photo luminescent materials. Moreover, the alkaline borosilicate minerals are widely used to fabricate LTCC materials such as SrO-, CaO–B2O3–SiO2 glass–ceramics, for the application in microwave devices and electronic packaging. However, until recently, a very little literature has reported the dielectric properties of the SrB2Si2O8 ceramics based on SrO–B2O3–SiO2 composition. More importantly, the preparation method of the single crystal phase ceramics is advantageous to develop an energy-saving process for the preparation of LTCC materials, since it does not need an additional glass manufacture process.
4 Conclusions
The present work is to discuss the sintering behavior of the SrB2Si2O8 ceramics based on SrO–B2O3–SiO2 composition, which are prepared by an aqueous solid-state process at a sintering temperature of 950 °C. It indicates that the SrO–B2O3 composition with low-melting characteristics can play an important role in the liquid-phase sintering of the SrB2Si2O8 ceramics. The phase formation and the achievement of the densification sintering are relative to the existence of a strontium borate hydrates in the ceramic slurries. At the same time, we realized that there are two obvious effects on the densification sintering of the SrB2Si2O8 ceramics, on the one hand, the existence of the BaO and B2O3 components in the SrB2Si2O8 ceramics can offer more possibilities to set a proper sintering behavior but on the other hand, the sintering behavior and the physical properties can be also deteriorated due to a high BaO, B2O3 or SiO2 content in the composition. The SrB2Si2O8 ceramics possess a dielectric constant of about 4 and its loss can be lowered up to 10−4.
References
Y. Imanaka, Multilayered low temperature co-fired ceramics (LTCC) technology (Springer, New York, 2005)
M. Eberstein, C. Glitzky, M. Gemeinert et al., Design of LTCC with high thermal expansion. Int J Ceram Technol 6, 1–8 (2009)
M. Eberstein, T. Rabe, W.A. Schiller, Influence of the glass phase on densification, microstructure, and properties of low-temperature co-fired ceramics. Int J Ceram Technol 3, 428–436 (2006)
D.N. Yoon, W.J. Huppmann, Grain growth and densification during liquid phase. Acta Metal 27, 693–698 (1979)
Song Chen, S.R. Zhang, X.H. Zhou, Thermal and dielectric properties of the LTCC composites based on the eutectic system BaO–Al2O3–SiO2–B2O3. J Mater Sci: Mater Electron 22, 453–457 (2011)
Song Chen, S.R. Zhang, X.H. Zhou, Phase formation and properties of the LTCC composite based on the eutectic system BaO–ZnO–SiO2–B2O3. J Alloys Compd 498, 185–190 (2010)
D. Thomas, M.T. Sebastian, Effect of Zn2+ substitution on the microwave dielectric properties of LiMgPO4 and the development of a new temperature stable glass free LTCC. J Eur Ceram Soc 32, 2359–2364 (2012)
S. Chen, D. Zhu, Phase formation and properties of BaO–B2O3–SiO2 and –Al2O3 ceramics prepared via an aqueous suspension route. J Alloys Compd 539, 73–79 (2012)
J.J. Bian, J.Y. Wu, Designing of glass-free LTCC microwave ceramic Ca1–x (Li0.5Nd0.5)xWO4 by crystal chemistry. J Am Ceram Soc 95, 318–323 (2012)
D. Dutta, M.P.F. Graca, M.A. Valente, S.K. Mendiratta, Structural characteristics and dielectric response of some zinc tellurite glasses and glass ceramics. Solid State Ionics 230, 66–71 (2013)
M.P. Saradhi, S. Boundin, U.V. Varadaraju, B. Raveau, A new BaB2Si2O8:Eu2+/Eu3+, Tb3+ phosphor- synthesis and photoluminescence properties. J Solid State Chem 182, 2496–2500 (2010)
Y. Wang, Z. Zhang, J. Zhang, Y. Lu, Electronic properties and rare earth ions photoluminescence behaviors in borosilicate: SrB2Si2O8. J Solid State Chem 183, 813–820 (2009)
H. Witzmann, G. Herzog, Luminescence-optical behavior of alkaline earth borate luminophors. Z Phys Chem 225, 197–208 (1964)
A.B. Meshalkin, A.B. Kaplun, Study of phase equilibria in system BaO–B2O3 from 32 to 67 mol% B2O3. J Cryst Growth 275, e301–e305 (2005)
German RM, Farooq S, Kipphut CM (1988) Mater. Kinetics of liquid sintering. Sci Eng A 105(106): 215–22
L.A. Pautov, A.A. Agakhanov, Maleevite, BaB2Si2O8, and Pekovite, SrB2Si2O8, new mineral species from the DARA-I-PIOZ alkaline massif, northern Tajikistan: description crystal structure. Can Mineral 42, 107–119 (2004)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Chen, S., Zhu, Dg., Sun, Pq. et al. Sintering behavior and dielectric properties of SrB2Si2O8 ceramics. J Mater Sci: Mater Electron 24, 4593–4599 (2013). https://doi.org/10.1007/s10854-013-1448-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-013-1448-z