Abstract
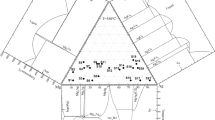
Although the Ag–Au–Pd system is crucial for several industrial applications and for the research on fundamental physics, no thermodynamic data on this ternary system at low temperatures have been reported in the literature. In the present study, activities of silver are directly measured by employing a solid-state EMF method, by using AgI as the solid electrolyte. The EMF was determined using a galvanic cell \( \left( - \right){\text{Pt}}\left| {\text{C}} \right.\left| {\text{Ag}} \right|{\text{AgI}}\left| {{\text{Ag-Au-Pd alloy}}} \right|{\text{C}}\left| {{\text{Pt}}\left( + \right)} \right. \), which produced novel experimental data on the thermodynamic properties of Ag–Au–Pd alloys. Darken method was used to calculate integral excess thermodynamic properties from the data. New thermodynamic characteristics, such as integral excess mixing Gibbs energy, entropy and enthalpy of the Ag–Au–Pd alloys, have been generated in a temperature range of 475 and 675 K. Isoactivity lines of silver in the system have been drawn throughout the Gibbs triangle. Thermodynamic properties of the binary Au–Pd alloys have been compared with the previous investigations.










Similar content being viewed by others
References
Ziya AB, Abbas T (2005) Kinetics of short-range order formation in the ternary Au–Ag–Pd alloys. Mater Chem Phys 91:442–446
Norman N, Warren BE (1951) X-Ray measurement of short range order in Ag–Au. J Appl Phys 22:483–486
Drost E, Hausselt J (1992) Uses of gold in jewellery. Interdiscip Sci Rev 17:271–280
Kempf B, Hausselt J (1992) Gold, its alloys and their uses in dentistry. Interdiscip Sci Rev 17:251–260
Kempf B, Schmauder S (1998) Thermodynamic modelling of precious metals alloys. Gold Bull 31:51–57
Fernández JL, Walsh DA, Bard AJ (2005) Thermodynamic guidelines for the design of bimetallic catalysts for oxygen electroreduction and rapid screening by scanning electrochemical microscopy. M–Co (M: Pd, Ag, Au). J Am Chem Soc 127:357–365
Niemi L, Minni E, Ivaska A (1986) An electrochemical and multispectroscopic study of corrosion of Ag–Pd–Cu–Au alloys. J Dent Res 65:888–891
Höhn R, Herzig C (1986) Direct determination of thermodynamic activities of gold in the systems gold–palladium and gold–silver–palladium. Z Metallkd 77:291–297
Nemilov VA, Rudnitsky AA, Vidusova TA (1946) Investigation of the Au–Pd–Ag system. Izvest Sekt Platiny 20:225–239
Miane JM, Gaune-Escard M, Bros JP (1977) Liquidus and solidus surfaces of the Ag–Au–Pd equilibrium phase diagram. High Temp High Press 9:465–469
Effenberg G, Ilyenko S (2006) Noble Metal Systems. Selected Systems from Ag–Al–Zn to Rh–Ru–Sc. Landolt–Börnstein—Group IV Physical Chemistry (Numerical Data and Functional Relationships in Science and Technology), vol 11B. Springer, Berlin, pp 50–52
Avarmaa K, Johto H, Taskinen P (2016) Distribution of precious metals (Ag, Au, Pd, Pt, and Rh) between copper matte and iron silicate slag. Metall Mater Trans B 47:244–255
Darken LS (1950) Application of the Gibbs–Duhem equation to ternary and multicomponent systems. J Am Chem Soc 72:2909–2914
Elliott JF, Chipman J (1951) The thermodynamic properties of liquid ternary cadmium solutions. J Am Chem Soc 73:2682–2693
Zheng M, Kozuka Z (1987) Thermodynamics study on liquid In–Bi–Pb ternary alloys. T Jpn I Met 28:925–933
Osadchii EG, Korepanov YI, Ionov KA (2011) Thermodynamic properties of Agx–Au1−x solid solution at 298–673 K and pressure of 1 atm, Vestn Otd nauk Zemle 3
White JL, Orr RL, Hultgren R (1957) The thermodynamic properties of silver–gold alloys. Acta Mater 5:747–760
Fischbach H (1980) EMF measurements on Ag–Au-alloys employing Ag-beta-alumina. Z Metallkund 71:448–450
Kubaschcwski O, Huchler O (1948) Über die Verwendung von Glas als Elektrolyt bei Messungen elektromotorischer Kräfte an Legierungen. Zur Thermochemie der Legierungen, Z Elektrochem 52:170–177
Feng D, Taskinen P (2014) Thermodynamic properties of silver–palladium alloys determined by a solid state electrochemical method. J Mater Sci 49:5790–5798. https://doi.org/10.1007/s10853-014-8310-4
Darby JB (1966) The relative heats of formation of solid gold–palladium alloys. Acta Mater 14:265–270
Pratt JN (1960) The thermodynamic properties of silver–palladium alloys. Trans Faraday Soc 56:975–987
Hayes FH, Kubaschewski O (1971) The heats of formation in the system gold–platinum–palladium. Met Sci J 5:37–40
Okamoto H, Massalski TB (1985) The Au − Pd (gold–palladium) system. Bull Alloy Phase Diagr 6:229–235
Tomiska J (1990) Computer-aided thermodynamics of solid Au–Pd alloys by knudsen cell mass spectrometry and calculation of the phase diagram. Z Metallkund 81:912–918
Sluiter MH, Colinet C, Pasturel A (2006) Ab initio calculation of the phase stability in Au–Pd and Ag–Pt alloys. Phys Rev B 73:174204
Aspiala M, Taskinen P (2016) Thermodynamic study of the Ag–Sb–Te system with an advanced EMF method. J Chem Thermodyn 93:261–266
Acknowledgements
The authors are indebted to Indonesian Government that provides the LPDP scholarship. The ARVI program of Finland (CLIC Innovation OY) for funding this research at Aalto University, Finland, is also greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santoso, I., Taskinen, P. Thermodynamic properties of Ag–Au–Pd alloys measured by a solid-state EMF method. J Mater Sci 53, 9232–9242 (2018). https://doi.org/10.1007/s10853-018-2189-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2189-4