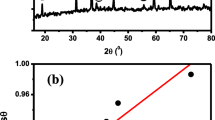
Abstract
The crystallization behavior of phosphate glasses of the composition 50P2O5–(40−x)CaO−xSrO–10Na2O with x = 0, 20, and 40 was investigated using non-isothermal differential thermal analysis. The study was performed on three different size fractions (fine powder (<45 μm), intermediate (300–500 μm), and coarse particles (>500 μm)). The DTA thermograms recorded for all glasses exhibited a single and symmetrical crystallization peak. The position and shape of the crystallization varies with the particles size. The activation energy for crystallization, E c, was calculated using equations proposed by Kissinger and Friedman. The calculated E c values were similar for both techniques. E c decreased with increasing the particle size for all glass compositions. The Johnson–Mehl–Avrami exponent n expressing the crystal growth dimensionality was quantified using the methods proposed by Augis–Bennett (AB) and Ozawa. In general, the AB method gave higher value than the Ozawa technique. For all glass composition the n value for the coarse powder suggested bulk crystallization whereas with decreasing particle size, the value of n indicated a complex surface crystallization. Glass monoliths were heat treated in electric oven at several temperatures for various times. XRD suggested crystallization of two phases: Ca(PO3)2 and NaCa(PO3)3 for the glass with x = 0, Sr(PO3)2 and NaSr(PO3)3 for the glass with x = 40, and a solid solution of (Ca,Sr)(PO3)2 and Na(Ca,Sr)(PO3)3 for the glass with x = 20. All crystals were found to preferentially nucleate and grow from the surface. Partially to fully crystallized glass particles were immersed in simulated body fluid for 24, 48, and 72 h. SEM/EDS of the particles and changes in the pH and ion concentrations of the solution (ICP-OES) suggested that crystallization prevents the CaP layer formation for all partially crystallized glasses.












Similar content being viewed by others
References
Stookey SD (1959) Catalyzed crystallization of glass in theory and practice. Ind Eng Chem Res 51:805–808
Hoeland W, Beal GH (eds) (2010) Glass-ceramic technology, 2nd edn. The American Ceramic Society, Wiley
Nakamura T, Yamamuro T, Higashi S, Kakutani Y, Kokubo T (1985) A new glass-ceramic for bone replacement: evaluation of its bonding to bone tissue. J Biomed Mater Res 19:685–698
Zanotto ED, Fokin VM (2003) Recent studies of internal and surface nucleation in silicate glasses. Philos Trans Math Phys Eng Sci 361:591–613
Davis MJ, Mitra I (2003) Crystallization measurements using DTA methods: applications to Zerodur®. J Am Ceram Soc 86:1540–1546
Massera J, Remond J, Musgraves J, Davis MJ, Misture ST, Petit L, Richardson K (2010) “Nucleation and growth behavior of glasses in the TeO2–Bi2O3–ZnO glass system”. J Non Cryst Solids 356:2947–2955
Ziani N, Belhadji M, Heireche L, Bouchaour Z, Belbachir M (2005) Crystallization kinetics of Ge20Te80Ge20Te80 chalcogenide glasses doped with Sb. Phys B 358:132–137
Joraid AA (2005) Limitation of the Johnson–Mehl–Avrami (JMA) formula for kinetic analysis of the crystallization of a chalcogenide glass. Thermochim Acta 436:78–82
Christian JW (1965) The theory of metals and alloys. Pergamon Press, Oxford
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem 29:1702–1706
Friedman HL (1963) Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. Polym Sci 6C:183–195
Ozawa T (1971) Kinetics of non-isothermal crystallization. Polymer 12:150–158
Augis JA, Bennett JE (1978) Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J Therm Anal Calorim 13:283–292
Marotta A, Buri A, Branda F (1980) Surface and bulk crystallization in non-isothermal devitrification of glasses. Thermochim Acta 40:397–403
Ray CS, Day DE (1980) Identifying internal and surface crystallization by differential thermal analysis for the glass-to-crystal transformations. Thermochim Acta 280/281:163–174
Malek J, Mitsuhashi T (2000) Testing method for the Johnson–Mehl–Avrami equation in kinetic analysis of crystallization processes. J Am Ceram Soc 83:2103–2105
Massera J, Fagerlund S, Hupa L, Hupa M (2012) Crystallization behavior of the commercial bioactive glasses 45S5 and S53P4. J Am Ceram Soc 95:607–613
Clement J, Manero JM, Planell JA (1999) Analysis of the structural changes of a phosphate glass during its dissolution in simulated body fluid. J Mater Sci Mater Med 10:729–732
Massera J, Petit L, Cardinal T, Videau JJ, Hupa M, Hupa L (2013) Thermal properties and surface reactivity in simulated body fluid of new strontium ion-containing phosphate glasses. J Mater Sci Mater Med 24:1407–1416
Starink MJ (2003) The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta 404:163–176
Gotor FJ, Criado JM (2001) Limitations of the Augis and Bennett method for kinetic analysis of the crystallization of glasses and conditions for correct use. J Am Ceram Soc 84:1797–1802
Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T (1990) Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A–W3. J Biomed Mater Res 24:721–734
Yinnon H, Uhlmann DR (1983) Applications of thermoanalytical techniques to the study of crystallization kinetics in glass-forming liquids, part I: theory. J Non Cryst Solids 54:253–275
Pacurariu C, Lazau RI, Lazau I, Tita D (2007) Kinetics of non-isothermal crystallization of some glass-ceramics based on basalt. J Therm Anal Calorim 88:647–652
Burnett DG, Douglas RW (1970) Liquid–liquid phase separation in the soda–lime–silica system. Phys Chem Glas 11:125–127
Massera J, Kokkari A, Närhi T, Hupa L SrO substituted CaO in bioactive phosphate-and silicate-based glasses: enhancement of gingival fibroblasts proliferation. J Mater Sci Mater Med (submitted)
Fagerlund S, Massera J, Moritz N, Hupa L, Hupa M (2012) Phase composition and in vitro bioactivity of porous implants made of bioactive glass S53P4. Acta Biomater 8:2331–2339
Acknowledgement
The Academy of Finland is gratefully acknowledged for the financial support of J. Massera.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Massera, J., Mayran, M., Rocherullé, J. et al. Crystallization behavior of phosphate glasses and its impact on the glasses’ bioactivity. J Mater Sci 50, 3091–3102 (2015). https://doi.org/10.1007/s10853-015-8869-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-8869-4