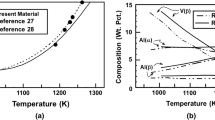
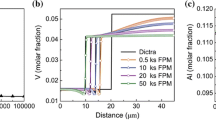
Abstract
An analytical model for dissolution kinetics of secondary phase particles upon isothermal annealing has been proposed. Considering the interactions of solute diffusion fields in front of the secondary phase/matrix interface upon dissolution, a Johnson–Mehl–Avrami type equation, subjected to necessary modification, was derived, in combination with a classic dissolution model for single-particle system. Compared with the semiempirical dissolution models, which are used to fit the experimental results and phase-field method simulation, the current model follows an analogous form, but with the time-dependent kinetic parameters. Distinct from the model fitting work published recently, the current model is derived from the diffusion-controlled transformation theory, while the modeling quality is guaranteed by the physically realistic model parameters. On this basis, the current model calculation leads to a clear relationship between the secondary phase volume fraction and the time. Accordingly, model predictions for isothermal θ′ dissolution in Al–3.0wt%–Cu alloy and silicon dissolution in Al–0.8wt%–Si alloy were performed; good agreement with the published experimental data has been achieved.








Similar content being viewed by others
Abbreviations
- C m :
-
Initial solute concentration in the matrix
- C α :
-
Solute concentration at the interface
- C β :
-
Solute concentration in the secondary phase
- D :
-
Diffusion coefficient of solute atoms
- D 0 :
-
Preexponential factor for diffusion
- f :
-
Volume fraction of the secondary phase
- f 0 :
-
Initial volume fraction of the secondary phase
- f eq :
-
Equilibrium volume fraction of the secondary phase
- f t :
-
Transformed fraction (transformation degree)
- k :
-
Dimensionless parameter related to solute concentrations, C β , C α , and C m
- K 0 :
-
Rate constant
- m :
-
Modified proportional factor
- n :
-
Transformed exponent
- Q :
-
Activation energy for dissolution
- Q D :
-
Activation energy for diffusion
- r d :
-
Decrement of the dissolving particle radius
- R :
-
Radius of the dissolving particle
- R 0 :
-
Initial radius of the dissolving particle
- t e :
-
The total transformation time needed for dissolution
- V e :
-
Extended transformed volume of the secondary phase
- x e :
-
Extended transformed fraction
References
Seiser B, Drautz R, Pettifor DG (2011) TCP phase predictions in Ni-based superalloys: structure maps revisited. Acta Mater 59:749–763
Lo KH, Shek CH, Lai JKL (2009) Recent developments in stainless steels. Mater Sci Eng R 65:39–104
Liu F, Cai Y, Guo XF, Yang GC (2000) Structure evolution in undercooled DD3 single crystal superalloy. Mater Sci Eng A 291:9–16
Fan K, Liu F, Liu XN, Zhang YX, Yang GC, Zhou YH (2008) Modeling of isothermal solid-state precipitation using an analytical treatment of soft impingement. Acta Mater 56:4309–4318
Clavaguera-Mora MT, Clavaguera N, Crespo D, Pradell T (2002) Crystallisation kinetics and microstructure development in metallic systems. Prog Mater Sci 47:559–619
Crespo D, Pradell T, Clavaguera-Mora MT, Clavaguera N (1997) Microstructure evaluation of primary crystallization with diffusion-controlled grain growth. Phys Rev B 55:3435–3444
Pradell T, Crespo D, Clavaguera N, Clavaguera-Mora MT (1998) Diffusion controlled grain growth in primary crystallization: Avrami exponents revisited. J Phys 10:3833–3844
Tkatch VI, Rassolov SG, Moiseeva TN, Popov VV (2005) Analytical description of isothermal primary crystallization kinetics of glasses: Fe85B15 amorphous alloy. J Non Cryst Solids 351:1658–1664
Chen YZ, Yang GC, Liu F, Liu N, Xie H, Zhou YH (2005) Microstrucure evolution in undercooled Fe-7.5 at% Ni alloys. J Cryst Growth 282:490–497
Liu N, Liu F, Yang GC, Chen YZ, Chen D, Yang CL, Zhou YH (2007) Grain refinement of undercooled single-phase Fe70Co30 alloys. Phys B 387:151–155
Ge Yu, Lai YKL, Zhang W (1997) Kinetics of transformation with nucleation and growth mechanism: diffusion-controlled reactions. J Appl Phys 82:4270–4276
Wang HF, Liu F, Zhang T, Yang GC, Zhou YH (2009) Kinetics of diffusion-controlled transformations: application of probability calculation. Acta Mater 57:3072–3083
Roncery LM, Weber S, Theisen W (2011) Nucleation and precipitation kinetics of M23C6 and M2N in an Fe–Mn–Cr–C–N austenitic matrix and their relationship with the sensitization phenomenon. Acta Mater 59:6275–6286
Zheng Y, Zheng YF, Jiang F, Li L, Yang H, Liu YN (2008) Effect of ageing treatment on the transformation behaviour of Ti–50.9 at.% Ni alloy. Acta Mater 56:736–745
Hewitt P, Butler EP (1986) Mechanisms and kinetics of θ′ dissolution in Al-3% Cu. Acta Metall 34:1163–1172
Tundal UH, Ryum N (1992) Dissolution of particles in binary alloys: Part II. Experimental investigation on an Al–Si alloy. Metall Trans A 23:445–449
Katona GL, Erdélyi Z, Beke DL, Dietrich Ch, Weigl F, Boyen HG, Koslowski B, Ziemann P (2005) Experimental evidence for a nonparabolic nanoscale interface shift during the dissolution of Ni into bulk Au(111). Phys Rev B 11:115432-1–115432-5
Milhet X, Arnoux M, Pelosin V, Colin J (2012) On the dissolution of the γ′ phase at the dendritic scale in a rhenium-containing nickel-based single crystal superalloy after high temperature exposure. Metall Mater Trans A 44:2031–2040
Thomas G, Whelan MJ (1961) Observations of precipitation in thin foils of aluminium +4 % copper alloy. Phil Mag 6:1103–1114
Aaron HB (1968) On the kinetics of precipitate dissolution. Metal Sci J 2:192–193
Whelan MJ (1969) On the kinetics of precipitate dissolution. Metal Sci J 3:95–97
Aaron HB, Fainstein D, Kotler GR (1970) Diffusion-limited phase transformations: a comparison and critical evaluation of the mathematical approximations. J Appl Phys 41:4404–4410
Nolfi FV Jr, Shewmon PG, Foster JS (1970) The dissolution kinetics of Fe3C in ferrite—a theory of interface migration. Metall Trans 1:2291–2298
Aaron HB, Kotler GR (1971) Second phase dissolution. Metall Trans 2:393–408
Enomoto M, Nojiri N (1997) Influence of interfacial curvature on the growth and dissolution kinetics of a spherical precipitate. Scripta Mater 36:625–632
Nojiri N, Enomoto M (1995) Diffusion-controlled dissolution of a spherical precipitate in an infinite binary alloy. Scripta Metall Mater 32:787–791
Brown LC (1976) Diffusion controlled dissolution of planar, cylindrical, and spherical precipitates. J Appl Phys 47:449–458
Tanzilli RA, Heckel RW (1968) Numerical solutions to the finite, diffusion-controlled, two phase, moving-interface problem (with planar, cylindrical, and spherical interfaces). Trans AIME 242:2313–2321
Tanzilli RA, Heckel RW (1969) A normalized treatment of the solution of second phase particles. Trans Met Soc AIME 245:1363–1366
Baty DL, Tanzilli RA, Heckel RW (1970) Solution kinetics of CuAl2 in an Al–4Cu alloy. Metall Trans 1:1651–1656
Tundal UH, Ryum N (1992) Dissolution of particles in binary alloys: Part I. Computer simulations. Metall Trans A 23:433–444
Vermolen F, Vuik K, van der Zwaag S (1998) A mathematical model for the dissolution kinetics of Mg2Si-phases in Al–Mg–Si alloys during homogenisation under industrial conditions. Mater Sci Eng A 254:13–32
Chen LQ (2002) Phase-field models for microstructure evolution. Annu Rev Mater Res 32:113–140
Moelans N, Blanpain B, Wollants P (2008) An introduction to phase-field modeling of microstructure evolution. Calphad 32:268–294
Javierre E, Vuik C, Vermolen FJ, Segal A (2007) A level set method for three dimensional vector Stefan problems: dissolution of stoichiometric particles in multi-component alloys. J Comput Phys 224:222–240
Chen LQ, Wang Y (1996) The continuum field approach to modeling microstructural evolution. JOM 48:13–18
Wang G, Xu DS, Ma N, Zhou N, Payton EJ, Yang R (2009) Simulation study of effects of initial particle size distribution on dissolution. Acta Mater 57:316–325
Ghosh G (2001) Dissolution and interfacial reactions of thin-film Ti/Ni/Ag metallizations in solder joints. Acta Mater 49:2609–2624
Chen Q, Ma N, Wu K, Wang Y (2004) Quantitative phase field modeling of diffusion-controlled precipitate growth and dissolution in Ti–Al–V. Scripta Mater 50:471–476
Kovacevic I, Sarler B (2005) Solution of a phase-field model for dissolution of primary particles in binary aluminum alloys by an r-adaptive mesh-free method. Mater Sci Eng A 413–414:423–428
Cormier J, Milhet X, Mendez J (2007) Effect of very high temperature short exposures on the dissolution of the γ′ phase in single crystal MC2 superalloy. J Mater Sci 42:7780–7786. doi:10.1007/s10853-007-1645-3
Giraud R, Hervier Z, Cormier J, Saint-Martin G, Hamon F, Milhet X, Mendez J (2012) Strain effect on the γ′ dissolution at high temperatures of a nickel-based single crystal superalloy. Metall Mater Trans A 44:131–146
Fukumoto S, Iwasaki Y, Motomura H, Fukuda Y (2012) Dissolution behavior of δ-ferrite in continuously cast slabs of SUS304 during heat treatment. ISIJ Int 52:74–79
Ferro P (2013) A dissolution kinetics model and its application to duplex stainless steels. Acta Mater 61:3141–3147
Mittemeijer EJ (1992) Review: analysis of the kinetics of phase transformations. J Mater Sci 27:3977–3987 10.1007/BF01105093
Christian JW (2002) The theory of transformations in melts and alloys. Pergamon, Oxford
Liu F, Sommer F, Mittemeijer EJ (2004) An analytical model for isothermal and isochronal transformation kinetics. J Mater Sci 39:1621–1634. doi:10.1023/B:JMSC.0000016161.79365.69
Liu F, Yang C, Yang G, Zhou Y (2007) Additivity rule, isothermal and non-isothermal transformations on the basis of an analytical transformation model. Acta Mater 55:5255–5267
Zhang RJ, Jing T, Jie WQ, Liu BC (2006) Phase-field simulation of solidification in multicomponent alloys coupled with thermodynamic and diffusion mobility databases. Acta Mater 54:2235–2239
Acknowledgements
The authors are grateful to the Natural Science Foundation of China (Nos. 51134011, 51101122, and 51071127), the National Basic Research Program of China (973 Program, No. 2011CB610403), China National Funds for Distinguished Young Scientists (No. 51125002), the Fundamental Research Fund (Nos. JC20120223), and the 111 Project (No. B08040) of Northwestern Polytechnical University.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Analogous to a mathematical treatment in Ref. [47], the extended transformed fraction is reformulated proceeding as follows.
For the two parts x e1 and x e2 in Eq. (34), two corresponding parameters b 1 and b 2 can be chosen in such a way that x e is due to only the first part,
Or that x e is due to only the second part,
With x e1′ = x e2′ = x e. Then, the total extended transformed fraction can be written as,
And
During dissolution, it can be seen that both x e1 and x e2 are positive and smaller than x e. Always two integers, a 1 and a 2, can be found to satisfy Eq. (35). Moreover, for integers, a 1 and a 2, it holds that \( a_{1} x_{{{\text{e}}1'}} = \sum\nolimits_{i = 1}^{{a_{1} }} {x_{{{\text{e}}1}} (i)} \) if x e1(1) = x e1(2) = …··· = x e1(a 1) = x e1′; and \( a_{2} x_{{{\text{e}}2{\prime }}} = \sum\nolimits_{i = 1}^{{a_{2} }} {x_{{{\text{e}}2}} (i)} \) if x e2(1) = x e2(2) = …··· = x e2(a 2) = x e2′. Then, taking both x e1(i) and x e2(i) equal to x e, Eq. (A.3) can be rewritten as,
Note that all fraction terms in Eq. (A.5) are equal. Substituting all x e1(i) and x e2(i) combined with Eqs. (A.1), (A.2) and (A.4), Eq. (A.5) becomes,
Rights and permissions
About this article
Cite this article
Zuo, Q., Liu, F., Wang, L. et al. An analytical model for secondary phase dissolution kinetics. J Mater Sci 49, 3066–3079 (2014). https://doi.org/10.1007/s10853-013-8009-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-013-8009-y