Abstract
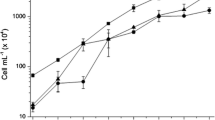
Thiamine release during synthetic mutualism between Chlorella sorokiniana co-immobilized in alginate beads with the microalgae growth-promoting bacterium Azospirillum brasilense was measured under stress conditions of pH, light intensity, and nitrogen starvation in short-term experiments. Thiamine release in the co-immobilized treatment was significantly higher at acidic pH compared to thiamine released by either microorganism alone. Under slightly alkaline pH, C. sorokiniana released the highest amount of thiamine. At stressful pH 6, the co-immobilized treatment released a higher quantity of thiamine than the sum of thiamine released by either microorganisms when immobilized separately. Release of thiamine by C. sorokiniana alone or co-immobilized was light intensity dependent; with higher the light intensity, more thiamine was released. Extreme light intensity negatively affected growth of the microalgae and release of thiamine. Nitrogen starvation during the first 24 h of culturing negatively affected release of thiamine by both microorganisms, where C. sorokiniana was more severely affected. Partial or continuous nitrogen starvation had similar negative effects on C. sorokiniana, but co-immobilization improved thiamine release. These results indicate that thiamine is released during synthetic mutualism between C. sorokiniana and A. brasilense, and this happens specifically during the alleviation of pH stress in the microalgae.



Similar content being viewed by others
References
Aaronson S, Dhawale SW, Patni NJ (1977) The cell content and secretion of water-soluble vitamins by several freshwater algae. Arch Microbiol 112:57–59
Bacilio M, Rodriguez H, Moreno M, Hernandez J-P, Bashan Y (2004) Mitigation of salt stress in wheat seedlings by a gfp-tagged Azospirillum lipoferum. Biol Fert Soils 40:188–193
Bar E, Rise M, Vishkautsan M, Arad SM (1995) Pigment and structural changes in Chlorella zofingiensis upon light and nitrogen stress. J Plant Physiol 146:527–534
Barea J-M, Pozo M-J, Azcón R, Azcón-Aguilar C (2013) Microbial interactions in the rhizosphere. In: de Brujin FJ (ed) Molecular microbial ecology of the rhizosphere. John Wiley & Sons, New York, pp 29–44
Bashan Y (1986) Alginate beads as synthetic inoculant carriers for slow release of bacteria that affect plant growth. Appl Environ Microbiol 51:1089–1098
Bashan Y (1999) Interactions of Azospirillum spp. in soils: a review. Biol Fert Soils 29:246–256
Bashan Y, de-Bashan LE (2010) How the plant growth-promoting bacterium Azospirillum promotes plant growth—a critical assessment. Adv Agron 108:77–136
Bashan Y, de-Bashan LE (2015) Inoculants for Azospirillum. In: Cassán F, Okon Y, Creus C (eds) Handbook for Azospirillum. Springer, Berlin, pp 469–485
Bashan Y, Holguin G (1994) Root-to-root travel of the beneficial bacterium Azospirillum brasilense. Appl Environ Microbiol 60:2120–2131
Bashan Y, Levanony H (1987) Horizontal and vertical movement of Azospirillum brasilense Cd in the soil and along the rhizosphere of wheat and weeds in controlled and field environments. J Gen Microbiol 133:3473–3480
Bashan Y, Levanony H, Klein E (1986) Evidence for a weak active external adsorption of Azospirillum brasilense Cd to wheat roots. J Gen Microbiol 132:3069–3073
Bashan Y, Puente ME, Rodriguez-Mendoza MN, Toledo G, Holguin G, Ferrera-Cerrato R, Pedrin S (1995) Survival of Azospirillum brasilense in the bulk soil and rhizosphere of 23 soil types. Appl Environ Microbiol 61:1938–1945
Bashan Y, Holguin G, de-Bashan LE (2004) Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances (1997–2003). Can J Microbiol 50:521–577
Bashan Y, Bustillos JJ, Leyva LA, Hernandez J-P, Bacilio M (2006) Increase in auxiliary photoprotective photosynthetic pigments in wheat seedlings induced by Azospirillum brasilense. Biol Fert Soils 42:279–285
Bashan Y, Trejo A, de-Bashan LE (2011) Development of two culture media for mass cultivation of Azospirillum spp. and for production of inoculants to enhance plant growth. Biol Fert Soils 47:963–969
Bashan Y, Lopez BR, Huss VAR, Amavizca E, de-Bashan LE (2015) Chlorella sorokiniana (formerly C. vulgaris) UTEX 2714, a non-thermotolerant microalga useful for biotechnological applications and as a reference strain. J Appl Phycol. doi:10.1007/s10811-015-0571-z
Baya AM, Boethling RS, Ramos-Cormenzana A (1981) Vitamin production in relation to phosphate solubilization by soil bacteria. Soil Biol Biochem 13:527–531
Berges JA, Charlebois DO, Mauzerall DC, Falkowski PG (1996) Differential effects of nitrogen limitation on photosynthetic efficiency of photosystems I and II in microalgae. J Plant Physiol 110:689–696
Bertrand EM, Allen AE (2012) Influence of vitamin B auxotrophy on nitrogen metabolism in eukaryotic phytoplankton. Front Microbiol 3:1–16. doi:10.3389/fmicb.2012.00375
Bronstein JL (1994) Our current understanding of mutualism. Q Rev Biol 69:31–51
Bucciarelli E, Sunda WG (2003) Influence of CO2, nitrate, phosphate, and silicate limitation on intracellular dimethylsulfoniopropionate in batch cultures of the coastal diatom Thalassiosira pseudonana. Limnol Oceanogr 48:2256–2265
Cassán F, Maiale S, Masciarelli O, Vidal A, Luna V, Ruiz O (2009) Cadaverine production by Azospirillum brasilense and its possible role in plant growth promotion and osmotic stress mitigation. Eur J Soil Biol 45:12–19
Cesco S, Mimmo T, Tonon G, Tomasi N, Pinton R, Terzano R, Neumann G, Weisskopf L, Renella G, Landi L, Nannipieri P (2012) Plant-borne flavonoids released into the rhizosphere: impact on soil bio-activities related to plant nutrition. A review. Biol Fert Soils 48:123–149
Choix FJ, de-Bashan LE, Bashan Y (2012a) Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: I. Autotrophic conditions. Enzyme Microb Technol 51:294–299
Choix FJ, de-Bashan LE, Bashan Y (2012b) Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense. II. Heterotrophic conditions. Enzyme Microb Technol 51:300–309
Choix FJ, Bashan Y, Mendoza A, de-Bashan LE (2014) Enhanced activity of ADP glucose pyrophosphorylase and formation of starch induced by Azospirillum brasilense in Chlorella vulgaris. J Biotechnol 177:22–34
Chrzanowski TH, Crotty RD, Hubbard JG, Welch RP (1984) Applicability of fluorescein diacetate method of detecting active bacteria in freshwater. Microb Ecol 10:179–185
Covarrubias SA, de-Bashan LE, Moreno M, Bashan Y (2012) Alginate beads provide a beneficial physical barrier against native microorganisms in wastewater treated with immobilized bacteria and microalgae. Appl Microbiol Biotechnol 93:2669–2680
Dahm H, Rózycki H, Strzelczyk E, Li CY (1993) Production of B-group vitamins by Azospirillum spp. grown in media of different pH at different temperatures. Zbl Mikrobiol 148:195–203
Dănet AF, Calatayud JM (1994) FIA-spectrophotometric determination of thiamine after UV-irradiation. Talanta 41:2147–2151
de-Bashan LE, Bashan Y (2008) Joint immobilization of plant growth-promoting bacteria and green microalgae in alginate beads as an experimental model for studying plant-bacterium interactions. Appl Environ Microbiol 74:6797–6802
de-Bashan LE, Bashan Y (2010) Immobilized microalgae for removing pollutants: review of practical aspects. Bioresour Technol 101:1611–1627
de-Bashan LE, Bashan Y, Moreno M, Lebsky VK, Bustillos JJ (2002) Increased pigment and lipid content, lipid variety, and cell and population size of the microalgae Chlorella spp. when co-immobilized in alginate beads with the microalgae-growth-promoting bacterium Azospirillum brasilense. Can J Microbiol 48:514–521
de-Bashan LE, Antoun H, Bashan Y (2005) Cultivation factors and population size control uptake of nitrogen by the microalgae Chlorella vulgaris when interacting with the microalgae growth-promoting bacterium Azospirillum brasilense. FEMS Microbiol Ecol 54:197–203
de-Bashan LE, Schmid M, Rothballer M, Hartmann A, Bashan Y (2011) Cell-cell interaction in the eukaryote-prokaryote model using the microalgae Chlorella vulgaris and the bacterium Azospirillum brasilense immobilized in polymer beads. J Phycol 47:1350–1359
de-Bashan LE, Hernandez J-P, Bashan Y (2015) Interaction of Azospirillum spp. with microalgae; a basic eukaryotic–prokaryotic model and its biotechnological applications. In: Cassán F, Okon Y, Creus C (eds) Handbook for Azospirillum. Springer, Berlin, pp 367–388
Doebeli M, Knowlton N (1998) The evolution of interspecific mutualisms. Proc Natl Acad Sci U S A 95:8676–8680
Floreto EAT, Teshima S, Ishikawa M (1996) Effects of nitrogen and phosphorus on the growth and fatty acid composition of Ulva pertusa Kjellman (Chlorophyta). Bot Mar 39:69–74
Gonzalez LE, Bashan Y (2000) Increased growth of the microalga Chlorella vulgaris when coimmobilized and cocultured in alginate beads with the plant-growth-promoting bacterium Azospirillum brasilense. Appl Environ Microbiol 66:1527–1531
Gonzalez LE, Cañizares RO, Baena S (1997) Efficiency of ammonia and phosphorus removal from a Colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and Scenedesmus dimorphus. Bioresour Technol 60:259–262
Hagen SR, Muneta P, Augustin J, LeTourneau D (1991) Stability and utilization of picloram, vitamins, and sucrose in a tissue culture medium. Plant Cell Tiss Org 25:45–48
Hartmann A, Schmid M, van Tuinen D, Berg G (2009) Plant-driven selection of microbes. Plant Soil 321:235–257
Imase M, Watanabe K, Aoyagi H, Tanaka H (2008) Construction of an artificial symbiotic community using a Chlorella-symbiont association as a model. FEMS Microbiol Ecol 63:273–282
Jurgenson CT, Begley TP, Ealick E (2009) The structural and biochemical foundations of thiamin biosynthesis. Annu Rev Biochem 78:569–603
Kadouri D, Jurkevitch E, Okon Y (2003) Involvement of the reserve material poly-β-hydroxybutyrate in Azospirillum brasilense stress endurance and root colonization. Appl Environ Microbiol 69:3244–3250
Kamilova F, Kravchenko LV, Shaposhnikov AI, Azarova T, Makarova N, Lugtenberg B (2006) Organic acids, sugars, and L-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol Plant-Microbe Interact 19:250–256
Kana TM, Geider RJ, Critchley C (1997) Regulation of photosynthetic pigments in micro-algae by multiple environmental factors: a dynamic balance hypothesis. New Phytol 137:629–638
Kessler A, Heil M (2011) The multiple faces of indirect defenses and their agents of natural selection. Funct Ecol 25:348–357
Khalil ZI, Asker MMS, El-Sayed S, Kobbia IA (2010) Effect of pH on growth and biochemical responses of Dunaliella bardawil and Chlorella ellipsoidea. World J Microbiol Biotechnol 26:1225–1231
Khozin-Goldberg I, Cohen Z (2011) Unraveling algal lipid metabolism: recent advances in gene identification. Biochimie 93:91–100
Lebeau T, Robert JM (2006) Biotechnology of immobilized micro algae: a culture technique for the future? In: Rao S (ed) Algal cultures, analogues of blooms and applications. Science Publishers, Enfield, pp 801–837
Levanony H, Bashan Y, Romano B, Klein E (1989) Ultrastructural localization and identification of Azospirillum brasilense Cd on and within wheat root by immuno-gold labeling. Plant Soil 117:207–218
Leyva LA, Bashan Y, Mendoza A, de-Bashan LE (2014) Accumulation of fatty acids in Chlorella vulgaris under heterotrophic conditions in relation to activity of acetyl-CoA carboxylase, temperature, and co-immobilization with Azospirillum brasilense. Naturwissenschaften 101:819–830
Leyva LA, Bashan Y, de-Bashan LE (2015) Activity of acetyl-CoA carboxylase is not directly linked to accumulation of lipids when Chlorella vulgaris is co-immobilised with Azospirillum brasilense in alginate under autotrophic and heterotrophic conditions. Ann Microbiol 65:339–349
Lv J-M, Cheng L-H, Xu X-H, Zhang L, Chen H-L (2010) Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour Technol 101:6797–6804
Meza B, de-Bashan LE, Bashan Y (2015a) Involvement of indole-3-acetic acid produced by Azospirillum brasilense in accumulating intracellular ammonium in Chlorella vulgaris. Res Microbiol 166:72–83
Meza B, de-Bashan LE, Hernandez J-P, Bashan Y (2015b) Accumulation of intra-cellular polyphosphate in Chlorella vulgaris cells is related to indole-3-acetic acid produced by Azospirillum brasilense. Res Microbiol 166:399–407
Momeni B, Chen C-C, Hillesland KL, Waite A, Shou W (2011) Using artificial systems to explore the ecology and evolution of symbioses. Cell Mol Life Sci 68:1353–1368
Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G, Valori F (2008) Effects of root exudates in microbial diversity and activity in rhizosphere soils. In: Nautiyal CS, Dion P (eds) Molecular mechanisms of plant and microbe coexistence. Springer, Heidelberg, pp 339–365
Nishijima T, Shiozaki R, Hata Y (1979) Production of vitamin B12, thiamine, and biotin by freshwater phytoplankton. Bull Jpn Soc Sci Fish 45:199–204
Nosaka K (2006) Recent progress in understanding thiamin biosynthesis and its genetic regulation in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 72:30–40
Paerl RW, Bertrand EM, Allen AE, Palenik B, Azam F (2015) Vitamin B1 ecophysiology of marine picoeukaryotic algae: strain-specific differences and a new role for bacteria in vitamin cycling. Limnol Oceanogr 60:215–228
Pal D, Khozin-Goldberg I, Cohen Z, Boussiba S (2011) The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl Microbiol Biotechnol 90:1429–1441
Palacios OA, Bashan Y, de-Bashan LE (2014) Proven and potential involvement of vitamins in interactions of plants with plant growth-promoting bacteria—an overview. Biol Fert Soils 50:415–432
Perez-Garcia O, Bashan Y (2015) Microalgal heterotrophic and mixotrophic culturing for bio-refining: From metabolic routes to techno-economics. In: Prokop A, Bajpai R, Zappi M (eds) Algal biorefineries II: products and biorefinery design. Springer International, Switzerland, (In Press). doi:10.1007/978-3-319-20200-6_3
Pratt R, Johnson E (1965) Production of thiamine, riboflavin, folic acid and biotin by Chlorella vulgaris and Chlorella pyrenoidosa. J Pharm Sci 54:871–874
Přibyl P, Cepák V, Zachleder V (2012) Production of lipids in 10 strains of Chlorella and Parachlorella, and enhanced lipid productivity in Chlorella vulgaris. Appl Microbiol Biotechnol 94:549–561
Puente ME, Holguin G, Glick BR, Bashan Y (1999) Root surface colonization of black mangrove seedlings by Azospirillum halofraeference and Azospirillum brasilense in seawater. FEMS Microbiol Ecol 29:283–292
Rodelas B, Salmerón V, Martinez-Toledo MV, González-López J (1993) Production of vitamins by Azospirillum brasilense in chemically-defined media. Plant Soil 153:97–101
Rodrigues AC, Bonifacio A, Antunes JEL, da Silveira JAG, Figueiredo MVB (2013) Minimization of oxidative stress in cowpea nodules by the interrelationship between Bradyrhizobium sp. and plant growth-promoting bacteria. Appl Soil Ecol 64:245–251
Rojas-Tapias DF, Bonilla RR, Dussán J (2012) Effect of inoculation with plant growth-promoting bacteria on growth and copper uptake by sunflowers. Water Air Soil Pollut 223:643–654
Sánchez-Machado DI, López-Cervantes J, López-Hernández J, Paseiro-Losada P (2004) Simultaneous determination of thiamine and riboflavin in edible marine seaweeds by high-performance liquid chromatography. J Chromatogr Sci 42:117–120
Schönwitz R, Ziegler H (1994) Exudation of water-soluble vitamins and of some carbohydrates by intact roots of maize seedlings (Zea mays L.) into a mineral nutrient solution. J Plant Physiol 107:7–14
Schyns G, Potot S, Geng Y, Barbosa TM, Henriques A, Perkins JB (2005) Isolation and characterization of new thiamine-deregulated mutants of Bacillus subtilis. J Bacteriol 187:8127–8136
Smith DJ, Underwood GJC (2000) The production of extracellular carbohydrates by estuarine benthic diatoms: the effect of growth phase and light and dark treatment. J Phycol 36:321–333
Smith AG, Croft MT, Moulin M, Webb ME (2007) Plants need their vitamins too. Curr Opin Plant Biol 10:266–275
Wagner-Döbler I, Ballhausen B, Berger M et al (2010) The complete genome sequence of the algal symbiont Dinoroseobacter shibae: a hitchhiker’s guide to life in the sea. ISME J 4:61–77
Widjaja A, Chien C-C, Ju Y-H (2009) Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J Taiwan Inst Chem E 40:13–20
Acknowledgments
At CIBNOR, we thank Manuel Moreno, Francisco Hernandez, and Juan-Pablo Hernandez for technical support and Ira Fogel for English and editorial suggestions. At Auburn University, we thank John Mcinroy for final English editing. Alejandro Palacios of the Autonomous University of Baja California Sur, La Paz, Mexico provided advice in statistical analysis. This study was supported by Consejo Nacional de Ciencia y Tecnologia of Mexico (CONACYT-Basic Science-2009, contract 164548) and time for writing by The Bashan Foundation, USA. O.A.P. was a recipient of a graduate fellowship (CONACYT 226169) and small periodic grants from the Bashan Foundation. This is contribution 2015-004 from the Bashan Institute of Science, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study is dedicated to the memory of the German/Spanish mycorrhizae researcher Dr. Horst Vierheilig (1964–2011) of CSIC, Spain
The term “release” stands for “extrusion”, “excretion”, and “produce”. A specific term is used when the exact mechanism is known.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Comparison of thiamine release by Chlorella sorokiniana and Azospirillum brasilense at different pH of the medium when immobilized separately or immobilized together in alginate beads (a, b, c) and growth of these microorganisms in these cultures (d, e, f). Values along curves denoted by different capital letters differ significantly using one-way ANOVA and LSD post hoc analysis at P <0.05. Points at each time interval denoted by different lower case letters differ significantly at P <0.05 by the same statistical analyses. Bars represent SE. (PDF 262 kb)
Fig. S2
Comparison of thiamine content in exudates under different light intensities released by Chlorella sorokiniana (a), Azospirillum brasilense (b) immobilized alone and co-immobilized (c) and comparison of growth of these microorganisms in these cultures (d, e, f). Values along curves denoted by different capital letters differ significantly using one-way ANOVA and LSD post hoc analysis at P <0.05. Points at each time interval denoted by different lower case letters differ significantly at P <0.05 by the same statistical analyses. Bars represent SE. (PDF 270 kb)
Rights and permissions
About this article
Cite this article
Palacios, O.A., Bashan, Y., Schmid, M. et al. Enhancement of thiamine release during synthetic mutualism between Chlorella sorokiniana and Azospirillum brasilense growing under stress conditions. J Appl Phycol 28, 1521–1531 (2016). https://doi.org/10.1007/s10811-015-0697-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0697-z