Abstract
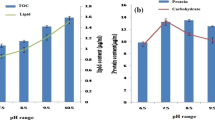
The goal of the present investigation was to study the effect of pH on growth and biochemical responses of Dunaliella bardawil and Chlorella ellipsoidea when exposed to different pH values. The two tested microalgae could grow in a wide range of pH (4–9 for D. bardawil and 4–10 for C. ellipsoidea). The dry weight gain and the biochemical components of D. bardawil were greatly enhanced at pH 7.5. In contrast, dry weight and carbohydrate content of C. ellipsoidea attained their maximum values at the alkaline pH. On the other hand, the protein content of C. ellipsoidea recorded its highest value at pH 4, while the pigment content of the same alga was highest at pH 4, 6, and 7.5 and decreased at alkaline pH. Both pH 6 and pH 9 stimulated the accumulation of β-carotene, vitamin E and vitamin C in D. bardawil, with the highest values of the three compounds recorded at pH 9. In the case of C. ellipsoidea, β-carotene content increased at pH 6 and pH 10 as compared with the control, but the amount of β-carotene was much higher at pH 6 than at pH 10. Vitamin E content was higher in C. ellipsoidea cells at pH 10 than at pH 6. Both pH 6 and pH 10 caused a significant decline in vitamin C content of C. ellipsoidea.






Similar content being viewed by others
References
Abalde J, Fabregas J, Herrero C (1991) β-Carotene, vitamin C and vitamin E content of the marine microalga Dunaliella tertiolecta cultured with different nitrogen sources. Bioresour Technol 38:121–125
Abe K, Nishimura N, Hirano M (1999) Simultaneous production of β-carotene, vitamin E and vitamin C by the aerial microalga Trentepohlia aurea. J Appl Phycol 11:331–336
Anon A (1983) Effect of pH on Dunaliella bardawil biomass and production of carotenoids. New Quarterly, Sanitary Engineering and Environmental Health Research Laboratory, Univ. California, Berkeley, p 33
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Azov Y (1982) Effect of pH on inorganic carbon uptake in algal cultures. Appl Environ Microbiol 43:1300–1306
Badger MR, Pfanz H, Budel B, Heber U, Lange OL (1993) Evidence for the functioning of photosynthetic CO2-concentrating mechanisms in lichens containing green algae and cyanobacterial photobions. Planta 191:57–70
Borowitzka MA, Borowitzka LJ (1988) Microalgal biotechnology. Cambridge University Press, Cambridge, p 466
Chitlaru E, Pick U (1991) Regulation of glycerol synthesis in response to osmotic changes in Dunaliella. Plant Physiol 96:50–60
Choo KS, Snoeijs P, Pedersén M (2004) Oxidative stress tolerance in the filamentous green algae Cladophora glomerata & Enteromorpha ahlneriana. J Exp Mar Biol Ecol 298:111–123
Christensen R (1996) Analysis of variance, design and regression, 1st edn. Chapman & Hall, London, p 587
Del Campo JA, Moreno J, Rodríguez H, Vargas MA, Rivas J, Guerrero MG (2000) Carotenoid content of chlorophycean microalgae: factors determining lute in accumulation in Muriellopsis sp. (Chlorophyta). J Biotechnol 76:51–59
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Dufosse L, Galaup P, Yaron A, Arad SM, Blanc P, Kotomballi N, Murthy C, Ravishankar GA (2005) Microorganisms and algae as sources of pigments and food use: a scientific oddity or an industrial reality? Trends Food Sci Technol 16:389–406
Eitenmiller RR, Landen WO (1999) Vitamin analysis for the health and food sciences. CRC Press, Boca Raton
Essa AM (1995) Osmoregulatory metabolites accumulated in halophilic algae grown on organic wastes. M.Sc., Cairo University, Egypt
Falkowski PG, Raven JA (1997) Aquatic photosynthesis. Blackwell, Malden, p 375
Foote CS, Denny RW, Weaver L, Chang Y, Peters J (1970) Quenching of singlet oxygen. Ann NY Acad Sci 171:139
Frank J (2005) Beyond vitamin E supplementation: an alternative strategy to improve vitamin E status. J Plant Physiol 162:834–843
Gertz C (1990) HPLC “tips &tricks” with over 1000 applications. Great Britian at the Alden Press, Oxford, p 608
Giao SM, Gonzalez-Snjose LM, Muniz P, Rivero-Perez MD (2008) Protection of deoxyribose and DNA from degradation by using aqueous extracts of several wild plants. J Sci Food Agric 88:633–640
Grace SC, Logan BA (1996) Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaves evergreen species. Plant Physiol 112:1631–1640
Hunter DJ, Willer WC (1994) Diet, body build, and breast cancer. Ann Rev Nutr 14:393–418
Imlay JA, Linn S (1988) DNA damage and oxygen radical toxicity. Science 240:1302–1309
Ip P-F, Chen F (2005) Production of astaxanthin by the green microalga Chlorella zofingiensis in the dark. Process Biochem 40:733–738
Kakizono T, Kakizono N, Nagai S (1993) Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococuus pluvialis. Appl Environ Microbiol 59:867–873
Liu BH, Lee YK (2000) Secondary carotenoids formation by the green alga Chlorococcum sp. J Appl Phycol 12:301–307
Loeblich LA (1972) Studies on the brine flagellate Dunaliella salina. Ph.D. Thesis, California University, San Diego
Loeblich LA (1982) Photosynthesis and pigments influenced by light intensity and salinity in the halophile Dunaliella salina (Chlorophyta). J Mar Biol Ass UK 62:493–508
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurements with Folin phenol reagent. J Biol Chem 193:265–275
Massyuk ND, Yurchenko VV (1962) Effect of hydrogen ion concentration on Dunaliella salina Teod. Ukranskya Bot Zhournal 19:91–95
Matsukawa R, Hotta M, Masuda Y, Chihara M, Karube I (2000) Antioxidants from carbon dioxide fixing Chlorella sorokiniana. J Appl Phycol 12:263–267
McLachlan J (1964) Some considerations of the growth of marine algae in artificial media. Can J Microbiol 10:769–782
Metzner H, Rau H, Senger H (1965) Untersuchungen zur synchronisier barkeit Einzelner pigmentmangel-Mutanten von Chlorella. Planta (Berl) 65:186–194
Mojaat M, Pruvost J, Foucault A, Legrand J (2008) Effect of organic carbon sources and Fe2+ ions on growth and β-carotene accumulation by Dunaliella salina. Biochem Eng J 39:177–184
Monteiro CM, Castro ML, Malcata FX (2009a) Use of the microalga Scenedesmus obliquus to remove cadmium cations from aqueous solutions. World J Microbiol Biotechnol 25:1573–1578
Monteiro CM, Marques APGC, Castro PML, Malcata FX (2009b) Characterization of Desmodesmus pleiomorphus isolated from a heavy metal-contaminated site: biosorption of zinc. Biodegradation 20:629–641
Nichols HW (1973) Growth media—fresh water. In: Stein JR (ed) Handbook of phycological method. Culture methods, growth measurements. Cambridge Univ Press, Cambridge, pp 7–24
Özyürek M, Bektaşoğlu B, Güçlü K, Güngör N, Apak R (2008) Simultaneous total antioxidant capacity assay of lipophilic and hydrophilic antioxidants in the same acetone-water solution containing 2% methyl-β-carotene-cyclodextrin using the cupric reducing antioxidant capacity (CUPRAC) method. Anal Chem Acta 630:28–39
Pelah D, Sintov A, Cohen E (2004) The effect of salt stress on the production of canthaxanthin and astaxanthin by Chlorella zofingiensis grown under limited light intensity. World J Microbiol Biotechnol 20:483–486
Pronina NA, Avramova S, Georgiev D, Semenenko VE (1981) A pattern of carbonic anhydrase activity in Chlorella and Scenedesmus on cell adaptation to high light intensity and low CO2 concentration. Fisiol Rastenii 28:43–52 (in Russian)
Su¨ltemeyer DF, Arnoroso G, Fock H (1995) Induction of intracellular carbonic anhydrases during the adaptation to low inorganic carbon concentrations in wild-type and ca-1 mutant cells of Chlamydomonas reinhardtii. Planta 196:217–224
Taha HM (2002) Comparative physiological and chemotaxonomical studies of some species of Dunaliella (volvocales). Ph.D. Faculty of Science, Alexandria University, pp 506
Ushimaru T, Nakagawa T, Fujioka Y, Daicho K, Natio M, Yamauchi Y, Nonaka H, Amako K, Yamawaki K, Murata N (2006) Transgenic Arabidopsis plants expressing the rice dehydroascorbate reductase gene are resistant to salt stress. J Plant Physiol 163:1179–1184
Vardi A, Berman-Frank I, Rozenberg T, Hadas O, Kaplan A, Levine A (1999) Programmed cell death of the dinoflagellate Peridinium gatunense is mediated by CO2 limitation and oxidative stress. Curr Biol 9:1061–1064
Wegmann K, Metzner H (1971) Synchronization of Dunaliella cultures. Arch Microbiol 78:360–367
Wistrand PJ (1984) Properties of membrane-bound carbonic anhydrase in biology and chemistry of the carbonic anhydrases. Ann NY Acad Sci 49:195–206
Zhang DH, Lee YK, Ng ML, Phang SM (1997) Composition and accumulation of secondary carotenoids in Chlorococcum sp. J Appl Phycol 9:147–155
Author information
Authors and Affiliations
Corresponding author
Additional information
Imam A. Kobbia—Late professor of Phycology.
Rights and permissions
About this article
Cite this article
Khalil, Z.I., Asker, M.M.S., El-Sayed, S. et al. Effect of pH on growth and biochemical responses of Dunaliella bardawil and Chlorella ellipsoidea . World J Microbiol Biotechnol 26, 1225–1231 (2010). https://doi.org/10.1007/s11274-009-0292-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-009-0292-z