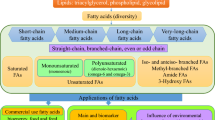
Abstract
Fatty acid biomarkers of the marine microalga Isochrysis zhangjiangensis in response to nitrogen depletion were identified by multivariate statistical analysis. Seven fatty acids (C16:0, C18:1n9, C18:4n3, C18:3n3, C18:5n3, C22:6n3, and C16:1n7) were selected and identified as potential biomarkers, among which C18:1n9 and C18:4n3 were enriched in the neutral lipids and glycolipids, respectively. Due to significant changes and specific distributions in different types of lipids, C18:1n9 and C18:4n3 were identified as biomarkers to quantify neutral lipid content. C18:1n9 was positively related with neutral lipid content, while C18:4n3 was negatively related with neutral lipid content. The correlation coefficients of C18:1n9 and C18:4n3 content in total fatty acids and neutral lipid content were 0.9960 and 0.9787, respectively. The good agreement between the neutral lipids content and fatty acid biomarkers content in total fatty acids indicates that neutral lipids can be quantified by specific fatty acids. Using fatty acid biomarkers to quantify neutral lipids requires much less sample analyzed by gas chromatography, which is a universal method, plus the simplicity of operation compared with conventional methods. The striking feature of the approach is individual fatty acid to represent the type of lipids. Therefore, applying fatty acid biomarkers to identify specific type of lipids promises to maximize lipid-related information and can be used to make further discoveries related to lipid metabolism.


Similar content being viewed by others
References
Alonso DL, Belarbi E-H, Rodríguez-Ruiz J, Segura CI, Giménez A (1998) Acyl lipids of three microalgae. Phytochemistry 47:1473–1481
Bigogno C, Khozin-Goldberg I, Boussiba S, Vonshak A, Cohen Z (2002) Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 60:497–503
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2012) The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresour Technol 124:217–226
Chen W, Sommerfeld M, Hu Q (2011) Microwave-assisted Nile red method for in vivo quantification of neutral lipids in microalgae. Bioresour Technol 102:135–141
Chen W, Zhang C, Song L, Sommerfeld M, Hu Q (2009) A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J Microbiol Meth 77:41–47
Dörmann P, Balbo I, Benning C (1999) Arabidopsis galactolipid biosynthesis and lipid trafficking mediated by DGD1. Science 284:2181–2184
Doan TTY, Obbard JP (2012) Enhanced intracellular lipid in Nannochloropsis sp. via random mutagenesis and flow cytometric cell sorting. Algal Res 1:17–21
Dunstan G, Volkman J, Barrett S, Garland C (1993) Changes in the lipid composition and maximisation of the polyunsaturated fatty acid content of three microalgae grown in mass culture. J Appl Phycol 5:71–83
Feng D, Chen Z, Xue S, Zhang W (2011) Increased lipid production of the marine oleaginous microalgae Isochrysis zhangjiangensis (Chrysophyta) by nitrogen supplement. Bioresour Technol 102:6710–6716
Griffiths M, van Hille R, Harrison S (2012) Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions. J Appl Phycol 24:989–1001
Hedenström M, Wiklund S, Sundberg B, Edlund U (2008) Visualization and interpretation of OPLS models based on 2D NMR data. Chemometr Intell Lab 92:110–117
Hofmann M, Eichenberger W (1997) Lipid and fatty acid composition of the marine brown alga Dictyopteris membranacea. Plant Cell Physiol 38:1046–1052
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Huerlimann R, de Nys R, Heimann K (2010) Growth, lipid content, productivity, and fatty acid composition of tropical microalgae for scale-up production. Biotechnol Bioeng 107:245–257
Jeffrey SW, LeRoi J-M (1997) Simple procedures for growing SCOR reference microalgal cultures. In: Jeffrey SW, Mantoura RFC, Wright SW (eds) Phytoplankton pigments in oceanography; monographs on oceanographic methodology 10. UNESCO, France, pp 181–205
Kind T, Meissen JK, Yang D, Nocito F, Vaniya A, Cheng Y-S, VanderGheynst JS, Fiehn O (2012) Qualitative analysis of algal secretions with multiple mass spectrometric platforms. J Chromatogr A 1244:139–147
Li X, Moellering ER, Liu B, Johnny C, Fedewa M, Sears BB, Kuo M-H, Benning C (2012) A galactoglycerolipid lipase is required for triacylglycerol accumulation and survival following nitrogen deprivation in Chlamydomonas reinhardtii. Plant Cell 24:4670–4686
Liu B, Vieler A, Li C, Jones AD, Benning C (2013) Triacylglycerol profiling of microalgae Chlamydomonas reinhardtii and Nannochloropsis oceanica. Bioresour Technol 146:310–316
Liu J, Sun Z, Zhong Y, Huang J, Hu Q, Chen F (2012) Stearoyl-acyl carrier protein desaturase gene from the oleaginous microalga Chlorella zofingiensis: cloning, characterization and transcriptional analysis. Planta 236:1665–1676
MacDougall K, McNichol J, McGinn P, O’Leary SB, Melanson J (2011) Triacylglycerol profiling of microalgae strains for biofuel feedstock by liquid chromatography–high-resolution mass spectrometry. Anal Bioanal Chem 401:2609–2616
Manandhar-Shrestha K, Hildebrand M (2013) Development of flow cytometric procedures for the efficient isolation of improved lipid accumulation mutants in a Chlorella sp. microalga. J Appl Phycol 25:1643–1651
Mansour MP, Volkman JK, Blackburn SI (2003) The effect of growth phase on the lipid class, fatty acid and sterol composition in the marine dinoflagellate. Gymnodinium sp. in batch culture. Phytochemistry 63:145–153
Merzlyak MN, Chivkunova OB, Gorelova OA, Reshetnikova IV, Solovchenko AE, Khozin-Goldberg I, Cohen Z (2007) Effect of nitrogen starvation on optical properties, pigments, and arachidonic acid content of the unicellular green alga Parietochloris incisa (Trebouxiophyceae, Chlorophyta). J Phycol 43:833–843
Msanne J, Xu D, Konda AR, Casas-Mollano JA, Awada T, Cahoon EB, Cerutti H (2012) Metabolic and gene expression changes triggered by nitrogen deprivation in the photoautotrophically grown microalgae Chlamydornonas reinhardtii and Coccomyxa sp. C-169. Phytochemistry 75:50–59
Ni Y, Su M, Lin J, Wang X, Qiu Y, Zhao A, Chen T, Jia W (2008) Metabolic profiling reveals disorder of amino acid metabolism in four brain regions from a rat model of chronic unpredictable mild stress. FEBS Lett 582:2627–2636
Ryckebosch E, Muylaert K, Foubert I (2012) Optimization of an analytical procedure for extraction of lipids from microalgae. J Am Oil Chem Soc 89:189–198
Siaut M, Cuine S, Cagnon C, Fessler B, Nguyen M, Carrier P, Beyly A, Beisson F, Triantaphylides C, Li-Beisson YH, Peltier G (2011) Oil accumulation in the model green alga Chlamydomonas reinhardtii: characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol 11:7. doi:10.1186/1472-6750-11-7
Solovchenko AE, Chivkunova OB, Semenova LR, Selyakh IO, Shcherbakov PN, Karpova EA, Lobakova ES (2013) Stress-induced changes in pigment and fatty acid content in the microalga Desmodesmus sp. Isolated from a White Sea hydroid. Russ J Plant Physiol 60:313–321
Su C-H, Chien L-J, Gomes J, Lin Y-S, Yu Y-K, Liou J-S, Syu R-J (2011) Factors affecting lipid accumulation by Nannochloropsis oculata in a two-stage cultivation process. J Appl Phycol 23:903–908
Sun MM, Sun J, Qiu JW, Jing H, Liu H (2012) Characterization of the proteomic profiles of the brown tide alga Aureoumbra lagunensis under phosphate-and nitrogen-limiting conditions and of its phosphate limitation-specific protein with alkaline phosphatase activity. Appl Environ Microb 78:2025–2033
Yoshida H, Yoshida N, Tomiyama-Sakamoto Y, Mizushina Y (2012) Characteristic distributions of fatty acids in different lipids from Jack beans (Canavalia gladiata DC.). Eur J Lipid Sci Tech 114:787–793
Acknowledgments
This work was supported by the Hundred Talent Program of the Chinese Academy of Sciences (No.A1097), National Key Basic Research Program of China “973 Program” (2011CBA00803), Liaoning Provincial Natural Science Foundation (2012010263), and Knowledge Innovation Programs of Dalian Institute of Chemical Physics, CAS (K2010A3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, HT., Yao, CH., Liu, YN. et al. Identification of fatty acid biomarkers for quantification of neutral lipids in marine microalgae Isochrysis zhangjiangensis . J Appl Phycol 27, 249–255 (2015). https://doi.org/10.1007/s10811-014-0300-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0300-z