Abstract
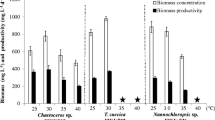
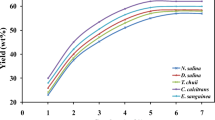
Microalgae are a promising alternative source of oil for biodiesel production. Identification of a species with desirable characteristics is a key component towards achieving economic feasibility for the process. This has been compromised by a lack of data allowing effective interspecies comparison. Eleven species of microalgae, selected on the basis of available literature data, were tested for lipid productivity, gravity sedimentation and the suitability of their fatty acid profiles for biodiesel production. The response to nitrogen limitation was species-specific. Lipid yields and productivity were higher at 150 mg L−1 nitrate than at 1,500 mg L−1 for all species tested except Spirulina platensis. The Chlorophyta, particularly Chlorella vulgaris and Scenedesmus, had the highest growth rates and showed the greatest increase in lipid content in response to nitrogen limitation. Cylindrotheca fusiformis, S. platensis, Scenedesmus and Tetraselmis suecica had the fastest settling rates and highest biomass recoveries after 24 h of gravity sedimentation. For most species, the fuel would need to be blended or culture conditions to be optimised to achieve the correct lipid profile in order for microalgal fuel to meet the European standards for biodiesel production (EN 14214). The most promising species overall were the freshwater algae Scenedesmus and C. vulgaris and the marine algae C. fusiformis and Nannochloropsis.





Similar content being viewed by others
References
Apt KE, Behrens PW (1999) Commercial developments in microalgal biotechnology. J Phycol 2:215–226
Ben-Amotz A, Tornabene TG, Thomas WH (1985) Chemical profile of selected species of microalgae with emphasis on lipids. J Phycol 1:72–81
Bold HC (1949) The morphology of Chlamydomonas chlamydogama sp. nov. Bull Torrey Bot Club 76:101–108
Borowitzka MA (1992) Algal biotechnology products and processes — matching science and economics. J Appl Phycol 4:267–279
Chelf P (1990) Environmental control of lipid and biomass production in two diatom species. J Appl Phycol 2:121–129
Chen F, Johns MR (1991) Effect of C/N ratio and aeration on the fatty acid composition of heterotrophic Chlorella sorokiniana. J Appl Phycol 3:203–209
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306.
Colla LM, Bertolin TE, Costa JAV (2004) Fatty acids profile of Spirulina platensis grown under different temperatures and nitrogen concentrations. Z Naturforsch C 1–2:55–59
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 6:1146–1151
Francisco E, Neves D, Jacob-Lopes E, Franco T (2010) Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality. J Chem Technol Biot 85:395–403
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 5:493–507
Griffiths MJ, van Hille RP, Harrison STL (2010) Selection of direct transesterification as the preferred method for assay of fatty acid content of microalgae. Lipids 11:1053–1060
Griffiths MJ, Garcin C, van Hille RP, Harrison STL (2011) Interference by pigment in the estimation of microalgal biomass concentration by optical density. J Microbiol Meth 85:119–123
Grobbelaar JU (2000) Physiological and technological considerations for optimising mass algal cultures. J Appl Phycol 12:201–206
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can J Microbiol 8:229–239
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 2:160–186
Harwood JL, Jones AL (1989) Lipid metabolism in algae. Adv Bot Res 16:1–53
Hsieh C, Wu W (2009) Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresour Technol 17:3921–3926
Illman A, Scragg AH, Shales SW (2000) Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb Technol 8:631–635
Knothe G (2009) Improving biodiesel fuel properties by modifying fatty ester composition. Energ Environ Sci 2:759–766
Lardon L, Hélias A, Sialve B, Steyer JP, Bernard O (2009) Life-cycle assessment of biodiesel production from microalgae. Environ Sci Technol 17:6475–6481
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 4:629–636
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 14(1):217–232
Miao X, Wu Q (2006) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 6:841–846
Patil V, Källqvist T, Olsen E, Vogt G, Gislerød HR (2006) Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquacult Int 1:1–9
Piorreck M, Baasch K, Pohl P (1984) Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue-green algae under different nitrogen regimes. Phytochemistry 2:207–216
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 6:635–648
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez A (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 1:261–268
Reitan KI, Rainuzzo JR, Olsen Y (1994) Effect of nutrient limitation on fatty acid and lipid content of marine microalgae. J Phycol 6:972–979
Renaud SM, Parry DL, Thinh L (1994) Microalgae for use in tropical aquaculture I: gross chemical and fatty acid composition of twelve species of microalgae from the northern territory, Australia. J Appl Phycol 3:337–345
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 1:100–112
Roessler P (1990) Environmental control of glycerolipid metabolism in microalgae: commercial implications and future research directions. J Phycol 26:393–399
Sheehan J, Dunahay T, Benemann JR, Roessler P (1998) A look back at the U.S. Department of Energy’s Aquatic Species Program: biodiesel from algae. Closeout report. National Renewable Energy Lab, Department of Energy, Golden, CO, USA. Report NREL/TP-580-24190
Shifrin NS, Chisholm SW (1981) Phytoplankton lipids: interspecific differences and effects of nitrate, silicate and light–dark cycles. J Phycol 17:374–384
Stansell G, Gray VM, Sym S (2011) Microalgal fatty acid composition: Implications for biodiesel quality. J Appl Phycol. doi:10.1007/s10811-011-9696-x
Stephenson AL, Dennis JS, Howe CJ, Scott SA, Smith AG (2010) Influence of nitrogen-limitation regime on the production by Chlorella vulgaris of lipids for biodiesel feedstocks. Biofuels 1:47–58
Suen Y, Hubbard JS, Holzer G, Tornabene TG (1987) Total lipid production of the green alga Nannochloropsis Sp. QII under different nitrogen regimes. J Phycol 23:289–296
Takagi M, Watanabe K, Yamaberi K, Yoshida T (2000) Limited feeding of potassium nitrate for intracellular lipid and triglyceride accumulation of Nannochloris Sp. UTEX LB1999. Appl Microbiol Biotechnol 1:112–117
Tong D, Hu C, Jiang K, Li Y (2010) Cetane number prediction of biodiesel from the composition of the fatty acid methyl esters. J Am Oil Chem Soc 88:415–423
Tornabene TG, Holzer G, Lien S, Burris N (1983) Lipid composition of the nitrogen starved green alga Neochloris oleoabundans. Enzyme Microb Technol 6:435–440
Volkman JK, Jeffrey S, Nichols PD, Rogers G, Garland C (1989) Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J Exp Mar Biol Ecol 3:219–240
Walne P (1970) Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria and Mytilus. HMSO, London
Xiong W, Li X, Xiang J, Wu Q (2008) High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl Microbiol Biotechnol 1:29–36
Zarrouk C (1966) Contribution à l’e étude d’une cyano-phycée. Influence de divers facteurs physiques et chimiques sur la croissance et la photosynthése de Spirulina maxima. PhD thesis, Université de Paris
Acknowledgements
The technical assistance of Christine Richardson and Frances Pocock is gratefully acknowledged. This work is based upon research supported by the South African National Energy Research Institute (SANERI) and the South African Research Chairs Initiative (SARChI) of the Department of Science and Technology and the National Research Foundation (NRF). The financial assistance of these organisations is hereby acknowledged. Opinions expressed and conclusions arrived at are those of the authors and are not necessarily to be attributed to SANERI, SARChI or the NRF.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Biomass concentration with time in five replicate cultures of Chlorella vulgaris. The average relative error between all five cultures across the growth cycle was <5% (PDF 23 kb)
Fig. S2
Biomass concentration (X) and lipid content (P) during a 14-day growth period for 11 species of microalgae under nitrogen replete (H, ●and ×) and limited (L, ○ and +) conditions (PDF 169 kb)
Fig. S3
Volumetric lipid content (PVOL) during a 14-day growth period for 11 species of microalgae under nitrogen replete (H, ●) and limited (L, ○) conditions (PDF 131 kb)
Fig. S4
Lipid productivity (QP) during a 14-day growth period for 11 species of microalgae under nitrogen replete (H, ●) and limited (L, ○) conditions (PDF 114 kb)
Rights and permissions
About this article
Cite this article
Griffiths, M.J., van Hille, R.P. & Harrison, S.T.L. Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions. J Appl Phycol 24, 989–1001 (2012). https://doi.org/10.1007/s10811-011-9723-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-011-9723-y