Abstract
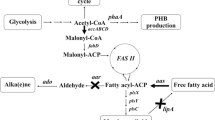
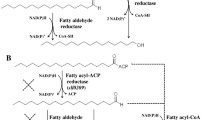
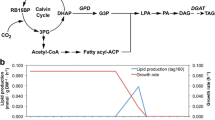
Photosynthetically derived fuels, such as those produced by microalgae, are touted as a future renewable energy source and a means for achieving energy independence. Realization of these claims, however, will require fuel production rates beyond the native capabilities of these microorganisms. The development of a metabolic engineering toolkit for microalgae will be key for reaching the production rates necessary for fuel production. This work advances the toolkit for cyanobacterial fuels by exploring the use of eukaryotic algal gene sources for free fatty acid biosynthesis rather than the traditional bacterial and plant sources. Many species of eukaryotic algae naturally accumulate high levels of triacylglycerol, a compound requiring three fatty acid side chains. Triacylglycerol accumulation implies that eukaryotic algae have naturally efficient enzymes for free fatty acid production, representing an unexplored resource for metabolic engineering targets. In this work, the model cyanobacterium, Synechococcus elongatus PCC7942, was engineered for free fatty acid production by targeting three main rate-limiting steps: (1) fatty acid release, catalyzed by a thioesterase, (2) fixation of carbon by ribulose-1,5-bisphosphate carboxylase/oxygenase, and (3) the first committed step in fatty acid biosynthesis, acetyl-CoA carboxylase. The recombinant acyl-ACP thioesterase and acetyl-CoA carboxylase were derived from the model green alga, Chlamydomonas reinhardtii CC-503. By targeting these proposed rate-determining steps, free fatty acid production was improved on a cell weight basis; however, the overall concentration of excreted free fatty acid did not increase. Recombinant gene expression was optimized by using native promoters, and while expression improved, the free fatty acid yield did not likewise increase. From physiological measurements, it was determined that free fatty acid production in S. elongatus PCC7942 is ultimately limited by the negative physiological effects associated with free fatty acid synthesis rather than bottlenecks within the metabolic pathway. This work demonstrates the successful expression of algal genes in a cyanobacterial host, but further improvement in free fatty acid yields will only be possible when the negative effects of free fatty acid production are mitigated.





Similar content being viewed by others
References
Atsumi S, Higashide W, Liao JC (2009) Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nature Biotech 27:1177–1180
Campbell D, Hurry V, Clarke AK, Gustafsson P, Öquist G (1998) Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol Mol Biol Rev 62:667–683
Cerutti H, Ma X, Msanne J, Repas T (2011) RNA-mediated silencing in algae: biological roles and tools for analysis of gene function. Eukaryot Cell 10:1164–1172
Cho H, Cronan JE (1995) Defective export of a periplasmic enzyme disrupts regulation of fatty acid synthesis. J Biol Chem 270:4216–4219
Cronan JE Jr, Waldrop GL (2002) Multi-subunit acetyl-CoA carboxylases. Progr Lipid Res 41:407–435
Davis MS, Solbiati J, Cronan JE (2000) Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. J Biol Chem 275:28593–28598
Dehesh K, Jones A, Knutzon DS, Voelker TA (1996) Production of high levels of 8:0 and 10:0 fatty acids in transgenic canola by overexpression of Ch FatB2, a thioesterase cDNA from Cuphea hookeriana. Plant J 9:167–172
Deng M-D, Coleman JR (1999) Ethanol synthesis by genetic engineering in cyanobacteria. Appl Environ Microbiol 65:523–528
Desbois A, Smith V (2010) Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol 85:1629–1642
Dunahay T, Jarvis E, Dais S, Roessler P (1996) Manipulation of microalgal lipid production using genetic engineering. Appl Biochem Biotechnol 57–58:223–231
Emanuelsson O, Nielsen H, von Heijne G (1999) ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci 8:978–984
DOE Joint Genome Institute Enabling Advances in Bioenergy and Environmental Research (2012) U.S. Department of Energy, Office of Science. Available at: http://www.jgi.doe.gov/. Accessed 23 August 2012
Fouquet R (2010) The slow search for solutions: lessons from historical energy transitions by sector and service. Energy Policy 38:6586–6596
Golden SS, Brusslan J, Haselkorn R (1987) Genetic engineering of the cyanobacterial chromosome. Methods Enzymol 153:215–231
Greene DL, Hopson JL, Li J (2006) Have we run out of oil yet? Oil peaking analysis from an optimist’s perspective. Energy Policy 34:515–531
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Jiang P, Cronan JE (1994) Inhibition of fatty acid synthesis in Escherichia coli in the absence of phospholipid synthesis and release of inhibition by thioesterase action. J Bacteriol 176:2814–2821
Liu S, Qureshi N (2009) How microbes tolerate ethanol and butanol. New Biotechnol 26:117–121
Liu X, Sheng J, Curtiss R III (2011) Fatty acid production in genetically modified cyanobacteria. Proc Nat Acad Sci 108:6899–6904
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Lu X, Vora H, Khosla C (2008) Overproduction of free fatty acids in E. coli: Implications for biodiesel production. Metab Eng 10:333–339
Ludwig M, Bryant DA (2012) Synechococcus sp. strain PCC 7002 transcriptome: acclimation to temperature, salinity, oxidative stress and mixotrophic growth conditions. Front Microbiol 3. doi:10.3389/fmicb.2012.00354
Magnuson K, Jackowski S, Rock CO, Cronan JE (1993) Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol Rev 57:522–542
Meng X, Yang J, Cao Y, Li L, Jiang X, Xu X, Liu W, Xian M, Zhang Y (2011) Increasing fatty acid production in E. coli by simulating the lipid accumulation of oleaginous microorganisms. J Ind Microbiol Biotechnol 38:919–925
Morariu V, Srinivasan B, Raykar V, Duraiswami R, Davis L (2008) Automatic online tuning for fast Gaussian summation. Adv Neural Inform Process Syst. Available at: http://sourceforge.net/projects/figtree/
NCBI: Genome (2012) National Center for Biotechnology Information, U.S. National Library of Medicine. Available at: http://www.ncbi.nlm.nih.gov/genome. Accessed 23 August 2012
Ohlrogge JB, Jaworski JG (1997) Regulation of fatty acid synthesis. Annu Rev Plant Physiol Plant Mol Biol 48:109–136
Peterhansel C, Niessen M, Kebeish RM (2008) Metabolic engineering towards the enhancement of photosynthesis. Photochem Photobiol 84:1317–1323
Pollard MR, Anderson L, Fan C, Hawkins DJ, Davies HM (1991) A specific acyl-ACP thioesterase implicated in medium-chain fatty acid production in immature cotyledons of Umbellularia californica. Arch Biochem Biophys 284:306–312
Radakovits R, Jinkerson RE, Darzins A, Posewitz MC (2010) Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell 9:486–501
Reed RH, Stewart WDP (1985) Osmotic adjustment and organic solute accumulation in unicellular cyanobacteria from freshwater and marine habitats. Mar Biol 88:1–9
Reppas NB, Ridley CP (2010) Methods and compositions for the recombinant biosynthesis of n-alkanes. US Patent No. 7794969
Ruffing AM, Jones HDT (2012) Physiological effects of free fatty acid production in genetically engineered Synechococcus elongatus PCC 7942. Biotechnol Bioeng 109:2190–2199
Sakamoto T, Bryant D (2002) Synergistic effect of high-light and low temperature on cell growth of the Δ12 fatty acid desaturase mutant in Synechococcus sp. PCC 7002. Photosynth Res 72:231–242
Salas JJ, Ohlrogge JB (2002) Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch Biochem Biophys 403:25–34
Tan X, Yao L, Gao Q, Wang W, Qi F, Lu X (2011) Photosynthesis driven conversion of carbon dioxide to fatty alcohols and hydrocarbons in cyanobacteria. Metab Eng 13:169–176
Tatsuzawa H, Takizawa E, Wada M, Yamamoto Y (1996) Fatty acid and lipid composition of the acidophilic green alga Chlamydomonas sp. J Phycol 32:598–601
Vijayan V, Jain IH, O’Shea EK (2011) A high resolution map of a cyanobacterial transcriptome. Genome Biol 12(5) doi:10.1186/gb-2011-12-5-r47
Voelker TA, Davies HM (1994) Alteration of the specificity and regulation of fatty acid synthesis of Escherichia coli by expression of a plant medium-chain acyl–acyl carrier protein thioesterase. J Bacteriol 176:7320–7327
Wu J-T, Chiang Y-R, Huang W-Y, Jane W-N (2006) Cytotoxic effects of free fatty acids on phytoplankton algae and cyanobacteria. Aquat Toxicol 80:338–345
Yu W-L, Ansari W, Schoepp N, Hannon M, Mayfield S, Burkart M (2011) Modifications of the metabolic pathways of lipid and triacylglycerol production in microalgae. Microb Cell Fact 10:91
Yuan L, Voelker TA, Hawkins DJ (1995) Modification of the substrate specificity of an acyl–acyl carrier protein thioesterase by protein engineering. Proc Nat Acad Sci 92:10639–10643
Acknowledgments
This work was supported by the Harry S. Truman Fellowship in National Security Science and Engineering and the Laboratory Directed Research and Development program. Sandia is a multi-program laboratory operated by Sandia Corporation, a Lockheed Martin Company, for the United States Department of Energy under Contract DE-ACO4-94AL85000. The author is grateful to Dr. James Laio (University of California, Los Angeles) for providing pSA126 and to the Kansas Lipidomics Research Center for performing GC/MS analysis on the extracted FFAs. Instrument acquisition and method development at the Kansas Lipidomics Research Center was supported by NSF grants MCB 0455318 and DBI 0521587, K-INBRE (NIH Grant P20 RR16475 from the INBRE program of the National Center for Research Resources), and NSF EPSCoR grant EPS-0236913 with matching support from the State of Kansas through Kansas Technology Enterprise Corporation and Kansas State University.
Conflict of interest
The author has no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruffing, A.M. Borrowing genes from Chlamydomonas reinhardtii for free fatty acid production in engineered cyanobacteria. J Appl Phycol 25, 1495–1507 (2013). https://doi.org/10.1007/s10811-013-9993-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-013-9993-7