Abstract
Purpose
To evaluate and follow-up of functional and morphological changes of the optic nerve and ocular structures prospectively in patients with early-stage Parkinson’s disease.
Materials and methods
Nineteen patients with a diagnosis of early-stage Parkinson’s disease and 19 age-matched healthy controls were included in the study. All participants were examined minimum three times at the intervals of at least 6 month following initial examination. Pattern visually evoked potentials (VEP), contrast sensitivity assessments at photopic conditions, color vision tests with Ishihara cards and full-field visual field tests were performed in addition to measurement of retinal nerve fiber layer (RNFL) thickness of four quadrants (top, bottom, nasal, temporal), central and mean macular thickness and macular volumes.
Results
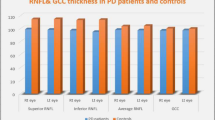
Best corrected visual acuity was observed significantly lower in study group within all three examinations. Contrast sensitivity values of the patient group were significantly lower in all spatial frequencies. P100 wave latency of VEP was significantly longer, and amplitude was lower in patient group; however, significant deterioration was not observed during the follow-up. Although average peripapillary RNFL thickness was not significant between groups, RNFL thickness in the upper quadrant was thinner in the patient group. While there was no difference in terms of mean macular thickness and total macular volume values between the groups initially, a significant decrease occurred in the patient group during the follow-up. During the initial and follow-up process, a significant deterioration in visual field was observed in the patient group.
Conclusion
Structural and functional disorders shown as electro-physiologically and morphologically exist in different parts of visual pathways in early-stage Parkinson’s disease.
Similar content being viewed by others
References
Archibald NK, Clarke MP, Mosimann UP et al (2009) The retina in Parkinson’s disease. Brain 132:1128–1145
Repka MX, Claro MC, Loupe DN et al (1996) Ocular motility in Parkinson’s disease. J Pediatr Ophthalmol Strabismus 33:144–147
Barnes J, David AS (2001) Visual hallucinations in Parkinson’s disease: a review and phenomenological survey. J Neurol Neurosurg Psychiatry 70:727–733
Biousse V, Skibell BC, Watts RL et al (2004) Ophthalmologic features of Parkinson’s disease [see comment]. Neurology 62:177–180
Sari ES, Koc R, Yazici A, Sahin G, Cakmak H, Kocaturk T, Ermis SS (2015) Tear osmolarity, break-up time and Schirmer’s scores in Parkinson’s disease. Turk J Ophthalmol 45:142–145
Goetz CG, Fan W, Leurgans S et al (2006) The malignant course of ‘benign hallucinations’ in Parkinson disease. Arch Neurol 63:713–716
Manuchehri K, Goodman S, Siviter L et al (2000) A controlled study of vigabatrin and visual abnormalities. Br J Ophthalmol 84:499–505
Moreno MC, Giagante B, Saidon P et al (2005) Visual defects associated üith vigabatrin: a study of epileptic Argentine patients. Can J Neurol Sci 32:459–464
De Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5:525–535
De Rijk MC, Breteler MM, Graveland GA et al (1995) Prevalence of Parkinson’s disease in the elderly: the Rotterdam study. Neurology 45:2143–2146
Satue M, Garcia-Martin E, Fuertes I et al (2013) Use of Fourier-domain OCT to detect retinal nerve fiber layer degeneration in Parkinson’s disease patients. Eye (Lond) 27:507–514
Archibald NK, Clarke MP, Mosimann UP et al (2011) Visual symptoms in Parkinson’s disease and Parkinson’s disease dementia. Mov Disord 26:2387–2395
Nowacka B, Lubinski W, Honczarenko K et al (2014) Ophthalmological features of Parkinson disease. Med Sci Monit 20:2243–2249
Stemplewitz B, Keserü M, Bittersohl D et al (2015) Scanning laser polarimetry and sprctral domain optical coherence tomography for the detection of retinal changes in Parkinson’s disease. Acta Ophthalmol 93:672–677
Sari ES, Koc R, Yazici A et al (2015) Ganglion cell-inner plexiform layer thickness in patients with Parkinson disease and association wiht disease severity and duration. J Neuroophthalmol 35:117–121
Kaur M, Saxena R, Singh D et al (2015) Correlation between structural and functional retinal changes in Parkinson disease. J Neuroophthalmol 35:254–258
Sacca SC, Bolognesi C, Battistella A et al (2009) Gene-environment interactions in ocular diseases. Mutat Res 66:98–117
Tsironi EE, Dastiridou A, Katsanos A et al (2012) Perimetric and retinal nerve fiber layer findings in patients with Parkinson’s disease. BMC Ophthalmol 12:54
Bayer AU, Keller ON, Ferrari F et al (2002) Association of glaucoma with neurodegenerative diseases with apoptotic cell death: Alzheimer’s disease and Parkinson’s disease. Am J Ophthalmol 133:135–137
Yenice O, Onal S, Midi I et al (2008) Visual field analysis in patients with Parkinson’s disease. Parkinsonism Relat Disord 14:193–198
McKinnon SJ (1997) Glaucoma, apoptosis, and neuroprotection. Curr Opin Ophthalmol 8:28–37
Langheinrich T, Tebartz van Elst L, Lagreze WA et al (2000) Visual contrast response functions in Parkinson’s disease: evidence from electroretinograms, visually evoked potentials and psychophysics. Clin Neurophysiol 111:66–74
Pieri V, Diederich NJ, Raman R et al (2000) Decreased colour discrimination and contrast sensitivity in Parkinson’s disease. J Neurol Sci 172:7–11
Miri S, Glazman S, Mylin L et al (2016) A combination of retinal morphology and visual electrophysiology testing increases diagnostic yield in Parkinson’s disease. Parkinsonism Relat Disord 22(Suppl 1):134–137
Kupersmith MJ, Shakin E, Siegel IM et al (1982) Visual system abnormalities in patients with Parkinson’s disease. Arch Neurol 39:284–286
Hutton JT, Morris JL, Elias JW et al (1991) Spatial contrast sensitivity is reduced bilaterally in Parkinson’s disease. Neurology 41:1200–1202
Buttner T, Muller T, Kuhn W (2000) Effects of apomorphine on visual functions in Parkinson’s disease. J Neural Transm 107:87–94
Birch J, Kolle RU, Kunkel M et al (1998) Acquired colour deficiency in patients with Parkinson’s disease. Vis Res 38:3421–3426
Oh YS, Kim JS, Chunq SW et al (2011) Color vision in Parkinson`s disease and essential tremor. Eur J Neurol 18:577–583
Reader TA, Quesney LF (1986) Dopamine in the visual cortex of the cat. Experientia 42:1242–1244
Dowling JE (1990) Functional and pharmacological organization of the retina: dopamine, interplexiform cells, and neuromodulation. In: Cohen B, Bodis-Wollner I (eds) Vision and the brain: the organization of the central visual system. Raven Press, New York, pp 1–18
Castelo-Branco M, Faria P, Forjaz V et al (2004) Simultaneous comparison of relative damage to chromatic pathways in ocular hypertension and glaucoma: correlation with clinical measures. Investig Ophthalmol Vis Sci 45:499–505
Campos SH, Forjaz V, Kozak LR et al (2005) Quantitative phenotyping of chromati dysfunction in best macular dystrophy. Arch Ophthalmol 123:944–949
Buttner TH, Kuhn W, Müller TH et al (1996) Chromatic and achromatic visual evoked potentials in Parkinson’s disease. Electroenceph Clin Neurophysiol 100:443–447
Dinner DS, Lüders H, Hanson M et al (1985) Pattern evoked potentials (PEPS) in Parkinson’s disease. Neurology 35:610–613
Barbato L, Rinalduzzi S, Laurenti M et al (1994) Color VEPs in Parkinson’s disease. Electroenceph Clin Neurophysiol 92:169–172
Altintas O, Iseri P, Ozkan B et al (2008) Correlation between retinal morphological and functional findings and clinical severity in Parkinson’s disease. Doc Ophthalmol 1116:137–146
Moschos MM, Tagaris G, Markopoulos I et al (2010) Morphologic changes and functional retinal impairment in patients with Parkinson disease without visual loss. Eur J Ophthalmol 21:24–29
Garcia-Martin E, Satue M, Fuertes I et al (2012) Ability and reproducibility of Fourier-domain optical coherence tomography to detect retinal nerve fiber layer atrophy in Parkinson’s disease. Ophthalmology 119:2161–2167
Kirbas S, Turkyilmaz K, Tufekci A et al (2013) Retinal nerve fiber layer thickness in Parkinson disease. J Neuroophthalmol 33:62–65
Garcia-Martin E, Rodrigues-Mena D, Satue M et al (2014) Electrophysiology and optical coherence tomography to evaluate Parkinson disease severity. Investig Ophthalmol Vis Sci 55:696–705
Yu JG, Feng YF, Xiang Y et al (2014) Retinal nerve fiber layer thickness changes in Parkinson disease: a meta- analysis. PLoS ONE 9(1):e85718
Aaker GD, Myung JS, Ehrlich JR et al (2010) Detection of retinal changes in Parkinson’s disease with spectral-domain optical coherence tomography. Clin Ophthalmol 4:1427–1432
Archibald NK, Clarke MP, Mosimann UP et al (2011) Retinal thickness in Parkinson’s disease. Parkinsonism Relat Disord 17:431–436
Albrecht P, Muller AK, Sudmeyer M et al (2012) Optical coherence tomography in parkinsonian syndromes. PLoS ONE 7:e34891
Shrier EM, Adam CR, Spund B et al (2012) Interocular asymmetry of foveal thickness in Parkinson disease. J Ophthalmol 2012:728457
Hajee M, March W, Lazzaro D et al (2009) Inner retinal layer thinning in Parkinson disease. Arch Ophthalmol 127:737–741
Adam C, Shrier E, Bodis-Wollner I et al (2013) Correlation of inner retinal thickness evaluated by spectral-domain optical coherence tomography and contrast sensitivity in Parkinson disease. J Neuroophthalmol 33:137–142
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Written informed consent was obtained from the subjects, and the study was conducted according to the tenets of the Declaration of Helsinki.
Rights and permissions
About this article
Cite this article
Hasanov, S., Demirkilinc Biler, E., Acarer, A. et al. Functional and morphological assessment of ocular structures and follow-up of patients with early-stage Parkinson’s disease. Int Ophthalmol 39, 1255–1262 (2019). https://doi.org/10.1007/s10792-018-0934-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-018-0934-y