Abstract
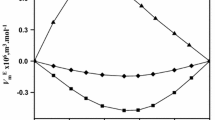
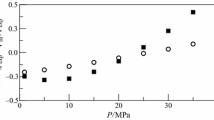
The presented work is a part of a research study on the volumetric, optical, and spectroscopic properties of binary mixtures containing glycerol with the four isomers of butanediol, namely: 1,2-butanediol or 1,3-butanediol or 1,4-butanediol or 2,3-butanediol. The density and refractive index measurements of pure components and their binary mixtures were carried out at atmospheric pressure and in a temperature range from 293.15 K to 318.15 K. The experimental data were then used to calculate for each system the following derived properties as a function of temperature and glycerol concentration: excess molar volumes, \(V^E\), partial molar volumes, \({\overline{V}}_i\), apparent molar volumes, \(V_{\phi i}\), partial molar volumes at infinite dilution, \({\overline{V}}_i^{\infty }\), excess partial molar volume at infinite dilution, \(\overline{V_i}^{E \infty }\), isobaric thermal expansions, \(\alpha\), excess thermal expansions, \(\alpha ^E\), and refractive index deviations, \(\Delta n _D\). Infrared spectroscopy analysis was also performed at atmospheric temperature and pressure. The experimental data obtained were fitted using the polynomial equation of Redlich-Kister. Excess molar volumes \(V^E\) for all the studied systems are negative over the entire composition range and at all the considered temperatures with deviations from ideality increasing with increasing temperature. The calculated molar excess properties were well correlated by the empirical Redlich-Kister polynomial. All measured and calculated properties reveal a significant influence of molecule structure, including the size, shape and position of the component hydroxyl groups. As intended, the infrared spectra of these binary mixtures display a high potential for hydrogen bonding. PC-SAFT EoS was successfully used to adjust the vapor pressure and liquid density of pure fluids, and was used predictively to correctly obtain the density of the mixture. Laplace’s rule was used to predict the refractive index of mixtures.





Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article and its supplementary information files.
References
P.C. Shukla, G. Belgiorno, G. Di Blasio, A.K. Agarwal, Alcohol as an alternative fuel for internal combustion engines (Springer, Singapore, 2021)
A. Basile, F. Dalena, Alcohols and bioalcohols: characteristics, production, and uses (Nova Science Publishers, Inc., New York, 2015)
M. Pagliaro, M. Rossi, The future of glycerol (Royal Society of Chemistry, Cambridge, 2010)
C.J. Mota, B.P. Pinto, A.L. de Lima, Glycerol: a versatile renewable feedstock for the chemical industry (Springer, Cham, 2017)
A. Mustain, E.D. Setiawati, R. Tetrisyanda, G. Wibawa, Experimental and predicted values of bubble point pressure for binary and ternary systems consisting of 1-butanol, 2-methyl-1-propanol, glycerol, and water. J. Chem. Eng. Data 67, 941–947 (2022)
V.B. Vicente, G.V. Olivieri, R.G. dos Santos, R.B. Torres, Volumetric properties of binary mixtures of glycerol+ alkanols (c1–c4): Experimental study and application of the peng–robinson–stryjek–vera equation of state. J. Chem. Thermodyn. 168, 106728 (2022)
M. Tyczyńska, A. Dentkiewicz, M. Jóźwiak, Thermodynamic and thermal analyze of n, n-dimethylformamide+ 1-butanol mixture properties based on density, sound velocity and heat capacity data. Molecules 28, 4698 (2023)
E.V. Anslyn, D.A. Dougherty, Modern physical organic chemistry (University science books, Sausalito, 2005)
G.I. Egorov, D.M. Makarov, Volumetric properties of binary mixtures of glycerol + tert-butanol over the temperature range 293.15 to 348.15 k at atmospheric pressure. J. Solut. Chem. 41, 536–554 (2012)
M.-L. Ge, J.-L. Ma, B. Chu, Densities and viscosities of propane-1, 2, 3-triol + ethane-1, 2-diol at t=(298.15 to 338.15) k. J. Chem. Eng. Data 55, 2649–2651 (2010)
B. Hawrylak, K. Gracie, R. Palepu, Thermodynamic properties of binary mixtures of butanediols with water. J. Solut. Chem. 27, 17–31 (1998)
Q.-S. Li, Y.-M. Tian, S. Wang, Densities and excess molar volumes for binary mixtures of 1, 4-butanediol+ 1, 2-propanediol,+ 1, 3-propanediol, and+ ethane-1, 2-diol from (293.15 to 328.15) k. J. Chem. Eng. Data 53, 271–274 (2008)
Y. Matsumoto, H. Touhara, K. Nakanishi, N. Watanabe, Molar excess enthalpies for water+ ethanediol,+ 1, 2-propanediol, and+ 1, 3-propanediol at 298.15 k. J. Chem. Thermodyn. 9, 801–805 (1977)
Anton Paar GmbH, Digital densimeter: Instuction manual DMA 5000, (2009)
K.R. Hall, D.J. Kirwan, O.L. Updike, Reporting precision of experiments. Chem. Eng. Educ. 9, 24–30 (1975)
J. Gross, G. Sadowski, Perturbed-chain saft: an equation of state based on a perturbation theory for chain molecules. Ind. Eng. Chem. Res. 40, 1244–1260 (2001)
J. Gross, G. Sadowski, Application of the perturbed-chain saft equation of state to associating systems. Ind. Eng. Chem. Res. 41, 5510–5515 (2002)
M. Almasi, A. Hernández, Experimental and theoretical studies of ethylene glycol dimethyl ether and 2-alkanol mixtures. Int. J. Thermophys. 44, 109 (2023)
A. Hernández, A.Z. Zeqiraj, F.R. Aliaj, Densities, sound speeds, and refractive indices of 1-propanol+ cyclohexane (or cyclohexene or cyclohexanone) binary mixtures at various temperatures under atmospheric pressure: experimental and modeling study. Int. J. Thermophys. 44, 102 (2023)
R. Abidi, M. Hichri, C. Lafuente, A. Hernández, Surface tensions for binary mixtures of alkyl levulinate+ alkanol: measurement and modeling. Int. J. Thermophys. 44, 33 (2023)
B. Giner, C. Lafuente, A. Villares, M. Haro, M.C. Lopez, Volumetric and refractive properties of binary mixtures containing 1, 4-dioxane and chloroalkanes. J. Chem. Thermodyn. 39, 148–157 (2007)
N.L. Benkelfat-Seladji, F. Ouaar, A. Hernández, N. Muñoz-Rujas, I. Bahadur, N.C.-B. Ahmed, E. Montero, L. Negadi, Measurements and modeling of physicochemical properties of pure and binary mixtures containing 1, 2-dimethoxyethane and some alcohols. J. Chem. Eng. Data 66, 3397–3416 (2021)
N.L. Benkelfat-Seladji, F. Ouaar, A. Hernández, I. Bahadur, N. Muñoz-Rujas, S.K. Singh, E. Montero, N.C.-B. Ahmed, L. Negadi, Density, speed of sound, refractive index of binary mixtures containing 2-ethoxyethanol and some alcohols: measurement and correlation. J. Chem. Thermodyn. 66, 106762 (2022)
O. Redlich, A.T. Kister, Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 345–348 (1948)
M.M. Alavianmehr, R. Ahmadi, N. Aguilar, M. El-Shaikh, S.M. Hosseini, S. Aparicio, Thermophysical and molecular modelling insights into glycerol+ alcohol liquid mixtures. J. Mol. Liq. 297, 111811 (2020)
H. Iloukhani, M. Almasi, Densities, viscosities, excess molar volumes, and refractive indices of acetonitrile and 2-alkanols binary mixtures at different temperatures: experimental results and application of the prigogine-flory-patterson theory. Thermochim. Acta 495, 139–148 (2009)
A.S. Alkindi, Y.M. Al-Wahaibi, A.H. Muggeridge, Physical properties (density, excess molar volume, viscosity, surface tension, and refractive index) of ethanol+ glycerol. J. Chem. Eng. Data 53, 2793–2796 (2008)
T.E. Daubert, R.P. Danner, Physical and thermodynamic properties of pure chemicals data compilation (Taylor & Francis, Bristol, 2004)
Acknowledgments
A.H acknowledges the economic support given by the UCSC.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
YC: Experimental investigation, Writing. AH: Theoretical investigation, Writing. FA: Experimental investigation, Writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chabouni, Y., Hernández, A. & Amireche, F. Volumetric, Optical and Spectroscopic Properties of Binary Mixtures of Glycerol with Butanediol Isomers. Int J Thermophys 44, 161 (2023). https://doi.org/10.1007/s10765-023-03272-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-023-03272-5