Abstract
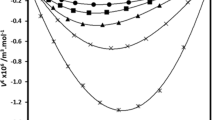
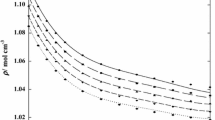
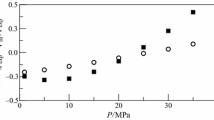
Densities, ρ, and viscosities, η, of pure isobutanol, 1-amino-2-propanol, and 1-propanol, along with their binary mixtures of {x 1isobutanol + x 21-propanol}, {x 11-amino-2-propanol + x 21-propanol}, and {x 11-amino-2-propanol + x 2isobutanol} were measured over the entire composition range and at temperatures (293.15–333.15) K at ambient pressure (81.5 kPa). Excess molar properties such as the excess molar volume, V Em , partial molar volumes, \( \bar{V}_{1} \) and \( \bar{V}_{2} \), excess partial molar volumes, \( \bar{V}_{1}^{\text{E}} \) and \( \bar{V}_{2}^{\text{E}} \), thermal expansion coefficient, α, excess thermal expansion coefficient, α E, viscosity deviation, Δη, and the excess Gibbs energy of activation, ∆G E*, for the binary mixtures were calculated from the experimental values of densities and viscosities. The excess values of the binary mixtures are negative in the entire composition range and at all temperatures, and increase with increasing temperature. Viscosity deviations, Δη, are negative over the entire composition range and decrease with increasing temperature. The viscosities of the mixtures were correlated by the models of McAllister, Heric, Hind, Katti, and Nissan. The obtained data were correlated by Redlich–Kister equation and the fitting parameters and standard deviations were determined.


Similar content being viewed by others
References
Pires, R.M., Costa, H.F., Ferreira, A.G.M., Fonseca, I.M.A.: Viscosity and density of water + ethyl acetate+ethanol mixtures at 298.15 and 318.15 K and atmospheric pressure. J. Chem. Eng. Data 52, 1240–1245 (2007)
Munoz, R., Burguet, M.C., Morlanes, N., Garcia-Usach, F.: Densities, refractive indices, and excess molar volumes of binary and ternary systems containing isobutyl alcohol, ethanol, 2-ethyl pentane, and methyl tert-butyl ether at 298.15 K. J. Chem. Eng. Data 45, 585–589 (2000)
Henni, A., Li, J., Tontiwachwuthikul, P.: Reaction kinetics of CO2 in aqueous 1-amino-2-propanol, 3-amino-1-propanol and dimethylmonoethanolamine solutions in temperature range of 298–313 K, using the stopped-flow technique. Ind. Eng. Chem. Res. 47, 2213–2220 (2008)
Krishna, T.V., Mohan, T.M.: Study of molecular interactions in the polar binary mixtures of N-methyl aniline and alcohols, using excess dielectric and thermodynamic parameters. J. Chem. Thermodyn. 47, 267–275 (2012)
Serbanovic, S.P., Kijevcanin, M.L., Radovic, I.R., Djordjevic, B.D.: Effect of temperature on the excess molar volumes of some alcohol + aromatic mixtures and modelling by cubic EOS mixing rules. Fluid Phase Equilib. 239, 69–82 (2006)
Dubey, G.P., Kumar, K.: Thermophysical properties of binary liquid mixtures of amine and alcohols at various temperatures. J. Chem. Thermodyn. 50, 7–14 (2012)
Cacela, C., Duarte, M.L., Fausto, R.: Structural and vibrational characterisation of 3-amino-1-propanol a concerted SCF-MO ab initio, Raman and infrared (matrix isolation and liquid phase) spectroscopy study. Spectrochim. Acta (A) 56, 1051–1064 (2000)
Dong, L., Chen, J., Gao, G.: Solubility of carbon dioxide in aqueous solutions of 3-amino-1-propanol. J. Chem. Eng. Data 55, 1030–1034 (2010)
Kermanpour, F., Niakan, H.Z., Sharifi, T.: Density and viscosity measurements of binary alkanol mixtures from (293.15 to 333.15) K at atmospheric pressure. J. Chem. Eng. Data 58, 1086–1091 (2013)
Dubey, G.P., Kumar, K.: Thermodynamic properties of binary mixtures of diethylene triamine with alcohols at different temperatures. Thermochim. Acta 524, 7–17 (2011)
Farhan, A.M., Awwad, A.M.: Densities, viscosities, and excess molar enthalpies of 2-pyrrolidone + butanol isomers at T = (293.15, 298.15, and 303.15) K. J. Chem. Eng. Data 54, 2095–2099 (2009)
Bravo-Sanchez, M.G., Iglesias-Silva, G.A., Estrada-Baltazar, A.: Densities and viscosities of binary mixtures of n-butanol with 2-butanol, isobutanol, and tert-butanol from (303.15 to 343.15) K. J. Chem. Eng. Data 55, 2310–2315 (2010)
Ali, A., Nain, K., Lal, B., Chand, D.: Densities, viscosities, and refractive indices of binary mixtures of benzene with isomeric butanols at 30 °C. Int. J. Thermophys. 25, 1835–1847 (2004)
Kermanpour, F., Niakan, H.Z.: Measurement and modeling the excess molar properties of binary mixtures of [C6mim][BF4] + 3-amino-1-propanol and {[C6mim][BF4] + isobutanol}: Application of Prigogine–Flory–Patterson theory. J. Chem. Thermodyn. 48, 129–139 (2012)
Kermanpour, F., Niakan, H.Z.: Experimental excess molar properties of binary mixtures of (3-amino-1-propanol + isobutanol, 2-propanol) at T = (293.15 to 333.15) K and modeling the excess molar volume by PFP theory. J. Chem. Thermodyn. 54, 10–19 (2012)
Mokraoui, H., Valtz, A., Coquelet, C., Richon, D.: Volumetric properties of the isopropanol amine–water mixture at atmospheric pressure from 283.15 to 353.15 K. Thermochim. Acta 440, 122–128 (2006)
Czerkas, S., Burczyk, A., Jadzyn, J., Stokhausen, M.: Viscosity of some propanols and amino propanols in mixtures with 1,4-dioxane. Phys. Chem. Liq. 37, 1–7 (1998)
Zarei, H.A., Shahvarpour, S.: Volumetric properties of binary and ternary liquid mixtures of 1-propanol (1) + 2-propanol (2) + water (3) at different temperatures and ambient pressure (81.5 kPa). J. Chem. Eng. Data 53, 1660–1668 (2008)
Zarei, H.A., Jalili, F.: Densities and derived thermoodynamic properties of (2-methoxy ethanol + 1-propanol, or 2-propanol, or 1,2-propandiol) at temperatures from T = (293.15 to 343.15) K. J. Chem. Thermodyn. 39, 55–66 (2007)
Wieser, M.E.: Atomic weights of the elements 2005 (IUPAC technical report). Pure Appl. Chem. 78, 2051–2066 (2006)
Wu, T.Y., Chen, B.K., Hao, L., Kuo, C.W., Sun, I.W.: Thermophysical properties of binary mixtures {1-methyl-3-pentylimidazolium tetrafluroborate + polyethylene glycol methyl ether}. J. Taiwan Inst. Chem. Eng. 43, 313–321 (2012)
Li, J.G., Hu, Y.F., Sun, F.S., Liu, Y.S., Liu, Z.C.: Densities and dynamic viscosities of the binary system (water + 1-hexyl-3-methylimidazolium bromide) at different temperatures. J. Chem. Thermodyn. 42, 904–908 (2010)
Anouti, M., Vigeant, A., Jacquemin, J., Brigouleix, C., Lemordant, D.: Volumetric properties, viscosity and refractive index of the protic ionic liquid, pyrrolidiniumoctanoate, in molecular solvents. J. Chem. Thermodyn. 42, 834–845 (2010)
McAllister, R.A.: The viscosity of liquid mixtures. AICHE 6, 427–431 (1960)
Heric, E.L.: On the viscosity of ternary mixtures. J. Chem. Eng. Data 11, 66–68 (1966)
Viswanath, D.S.: Viscosity of Liquids, Theory, Estimation, Experiment, and Data. Springer, Netherland (2007)
Oswal, S.L., Cheewala, D.B., Prajapati, K.D., Gradas, R.L., Ghael, N.Y., Ijardar, S.P.: Speeds of sound, isentropic compressibilities, viscosities, and excess molar volumes of binary mixtures of alkanoates with tetra- and trichloromethanes at 303.15 K. Thermochim. Acta 426, 141–149 (2005)
Redlich, O., Kister, A.T.: Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 345–348 (1948)
Acknowledgement
The research council of Bu-Ali Sina University is appreciated for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kermanpour, F., Ettefagh, T.S. & Iloukhani, H. Measurement and Calculation the Excess Molar Properties of Binary Mixtures Containing Isobutanol, 1-Amino-2-Propanol, and 1-Propanol at Temperatures of (293.15 to 333.15) K. J Solution Chem 46, 446–460 (2017). https://doi.org/10.1007/s10953-017-0580-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-017-0580-4