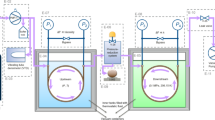
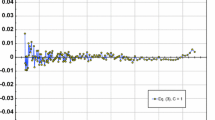
The zero-density viscosity \({\eta_{0,T}^{\rm gas}}\) of hydrogen, methane, and argon was determined in the temperature range from 200 to 400 K, with standard uncertainties of 0.084% for hydrogen and argon and 0.096% for methane. These uncertainties are dominated by the uncertainty of helium’s viscosity \({\eta_{0,T}^{\rm He}}\) , which we estimate to be 0.080% from the difference between ab initio and measured values at 298.15 K. For xenon, measurements ranged between 200 and 300 K and the zero-density viscosity \(\eta _{0,T}^{\rm Xe} \) was determined with an uncertainty of 0.11%. The data imply that xenon’s viscosity virial coefficient is positive over this temperature range, in contrast with the predictions of corresponding-states models. Furthermore, the xenon data are inconsistent with Curtiss’ prediction that bound pairs cause an anomalous viscosity decrease at low reduced temperatures. At 298.15 K. the ratios \(\eta _{0,298}^{\rm Ar}\!/\eta_{0,298}^{\rm He} ,{\eta _{0,298}^{{\rm CH}_{4}} }\!/\eta_{0,298}^{\rm He },{\eta _{0,298}^{{\rm H}_2} }\!/{\eta_{0,298}^{\rm He}},{\eta_{0,298}^{\rm Xe} }\!/{\eta _{0,298}^{\rm He} }, {\eta _{0,298}^{{\rm N}_2} }\!/{\eta _{0,298}^{\rm He}}\) , and \({\eta _{0,298}^{{\rm C}_2{\rm H}_6} }/{\eta _{0,298}^{\rm He} }\) were determined with a relative uncertainty of less than 0.024% by measuring the flow rate of these gases through a quartz capillary while simultaneously measuring the pressures at the ends of the capillary. Between 200 and 400 K, a two-capillary viscometer was used to determine \({\eta_{0,T}^{\rm gas} }/{\eta_{0,T}^{\rm He} }\) with an uncertainty of 0.024% for H2 and Ar, 0.053% for CH4, and 0.077% for Xe. From \({\eta_{0,T}^{\rm gas} }/{\eta_{0,T}^{\rm He} }, \eta_{0,T}^{\rm gas} \) was computed using the values of \(\eta_{0,T}^{\rm He}\) calculated ab initio. Finally, the thermal conductivity of Xe and Ar was computed from \(\eta_{0,T}^{\rm gas} \) and values of the Prandtl number that were computed from interatomic potentials. These results may help to improve correlations for the transport properties of these gases and assist efforts to develop ab initio two- and three-body intermolecular potentials for these gases. Reference viscosities for seven gases at 100 kPa are provided for gas metering applications.
Similar content being viewed by others
References
May E.F., Moldover M.R., Berg R.F., Hurly J.J. (2006) Metrologia 43:247
Moldover M.R., Boyes S.J., Meyer C.W., Goodwin A.R.H. (1999) J. Res. NIST 104:11
G. F. Strouse, D. R. Defibaugh, M. R. Moldover, and D. C. Ripple, in Temperature: Its Measurement and Control in Science and Industry, Vol. VII, 8th Int. Temp. Symp., D. C. Ripple, ed. (American Institute of Physics, New York, 2003), pp. 31–36.
Moldover M.R., Trusler J.P.M., Edwards T.J., Mehl J.B., Davis R.S. (1988) J. Res. Natl. Bur. Stand. (U.S.) 93:85
B. Fellmuth, J. Fischer, C. Gaiser, and W. Buck, BIPM Document CCT/05-02, http://www.bipm.fr/cc/CCT/Allowed/23/CCT_05_02.pdf (2005).
Curtiss C.F. (1992) J. Chem. Phys. 97:7679
Hirschfelder J.O., Curtiss C.F., Bird R.B. (1964) Molecular Theory of Gases and Liquids. Wiley, New York
Dham A.K., Meath W.J., Allnat A.R., Aziz R.A., Slaman M.J. (1990) Chem. Phys. 142:173
See, for example, R. Hellman, E. Bich, and E. Vogel, Proc. 16th Symp. Thermophys. Props., Boulder, Colorado (July 31–August 4, 2006).
Hurly J.J., Moldover M.R. (2000) . J. Res. Natl. Inst. Stand. Technol. (U.S.) 105:667
J. J. Hurly and M. R. Moldover, unpublished (2004); J. J. Hurly and J. B. Mehl, J. Res. Natl. Inst. Stand. Technol. 112:75 (2007).
Kestin J., Leidenfrost W. (1959) Physica 25:1033
Evers C., Lösch H.W., Wagner W. (2002) Int. J. Thermophys. 23:1411
Berg R.F. (2005) Metrologia 42:11
R. F. Berg, erratum to Metrologia 42:11 (2005); Metrologia 43:183 (2006).
Berg R.F., Tison S.A., (2004) . J. Res. Natl. Inst. Stand. Technol. (U.S.) 109:435
Boyes S.J. (1994) Chem. Phys. Lett. 221:467
Hurly J.J., Schmidt J.W., Boyes S.J., Moldover M.R. (1997) Int. J. Thermophys. 18:579
Vogel E., Wilhelm J., Küchenmeister C., Jaeschke M. (2000) High Temp. High Press. 32:73
E. W. Lemmon, M. O. McLinden, and M. L. Huber, Reference Fluid Thermodynamic and Transport Properties, NIST Standard Reference Database 23, Version 7.0, http://www.nist.gov/srd/nist23.htm (2002).
Assael M.J., Mixafendi S., Wakeham W.A. (1986) J. Phys. Chem. Ref. Data 15:1315
Rainwater J.C., Friend D.G. (1987) Phys. Rev. A 36:4062
Lemmon E.W., Jacobsen R.T. (2004) Int. J. Thermophys. 25:21
Gracki J.A., Flynn G.P., Ross J. (1969) J. Chem. Phys. 51:3856
Wilhelm J., Vogel E., (2000) Int. J. Thermophys. 21:301
Vogel E., Küchenmeister C., Bich E., Laesecke A. (1998) J. Phys. Chem. Ref. Data 27:947
Hurly J.J., Gillis K.A., Mehl J.B., Moldover M.R. (2003) Int. J. Thermophys. 24:1441
Kestin J., Yata J. (1968) J. Chem. Phys. 49:4780
Schley P., Jaeschke M., Küchenmeister C., Vogel E. (2004) Int. J. Thermophys. 25:1623
Barua A.K., Afzal M., Flynn G.P., Ross J. (1964) J. Chem. Phys. 41:374
Hendl S., Vogel E. (1994) Fluid Phase Equilib. 76:259
Najafi B., Ghayeb Y., Rainwater J.C., Alavi S., Snider R.F. (1998) Physica A 260:31
Clarke A.G., Smith E.B. (1968) J. Chem. Phys. 48:3988
Clarke A.G., Smith E.B. (1969) J. Chem. Phys. 51:4156
Flynn G.P., Hanks R.V., Lemaire N.A., Ross J. (1963) J. Chem. Phys. 38:154
Bich E., Millat J., Vogel E. (1990) J. Phys. Chem. Ref. Data 19:1289
Michels A., Wassenaar T., Louwerse P. (1954) Physica 20:99
Vogel E. (1984) Ber. Bunsenges. Phys. Chem. 88:997
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
May, E.F., Berg, R.F. & Moldover, M.R. Reference Viscosities of H2, CH4, Ar, and Xe at Low Densities. Int J Thermophys 28, 1085–1110 (2007). https://doi.org/10.1007/s10765-007-0198-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-007-0198-7