Abstract
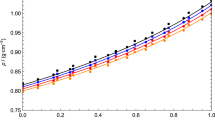
Low-density viscosity measurements on eight gaseous and vapor mixtures between 297 K and 638 K, originally performed using oscillating-disk viscometers, were re-evaluated after improved re-calibration. The relative combined expanded (\(k=2\)) uncertainty of the re-evaluated data are 0.2 % near room temperature and increases to 0.3 % at higher temperatures. The re-evaluated data were converted into quasi-isothermal viscosity data. Those for carbon dioxide–ethane, propane–isobutane, and methanol–triethylamine could be used to determine the zero-density and initial density viscosities, \(\eta _\text{mix}^{(0)}\) and \(\eta _\text{mix}^{(1)}\). The \(\eta _\text{mix}^{(0)}\) data for carbon dioxide–ethane agree almost perfectly with viscosity values theoretically computed for the nonspherical potential of the intermolecular interaction. Three procedures were applied to determine the interaction viscosity, \(\eta _{ij}^{(0)}\), and the product of molar density and diffusion, \((\rho D_{ij})^{(0)}\), both in the limit of zero density. In a first procedure only applicable for the three mentioned mixtures, \(\eta _{ij}^{(0)}\) values were derived from the \(\eta _\text{mix}^{(0)}\) data additionally requiring \(A_{ij}^*\) values (ratio between effective cross sections of viscosity and diffusion). This procedure should provide the best results when it is possible to use \(A_{ij}^*\) values computed for the nonspherical potential. This was only feasible for carbon dioxide–ethane, for which the experimentally based \(\eta _{ij}^{(0)}\) and \((\rho D_{ij})^{(0)}\) data perfectly agree with theoretically calculated values. For the seven other mixtures, the resulting data represent only preliminary ones. The second and third procedures were applied to the six vapor mixtures methanol with triethylamine, benzene, and cyclohexane and benzene with toluene, p-xylene, and phenol. The resulting data showed a density dependence and were extrapolated to zero density.






Similar content being viewed by others
Data Availability
The used datasets are in the manuscript. But if researchers are interested in further intermediate data, they can ask the author to get this information.
References
J. Kestin, E. Paykoç, J.V. Sengers, Physica 83, 271 (1971)
J. Kestin, S.T. Ro, W.A. Wakeham, J. Chem. Phys. 56, 4119 (1972)
E. Vogel, B. Jäger, R. Hellmann, E. Bich, Mol. Phys. 108, 3335 (2010)
R.F. Berg, M.R. Moldover, J. Phys. Chem. Ref. Data 41, 043104 (2012)
R. Hellmann, Mol. Phys. 111, 387 (2013)
E. Vogel, J. Phys. Chem. Ref. Data 49, 043102 (2020)
E. Vogel, E. Bich, Int. J. Thermophys. 42, 153 (2021)
S. Hendl, E. Vogel, High Temp.-High Press. 25, 279 (1993)
C. Küchenmeister, E. Vogel, J. Baranski, High Temp.-High Press. 33, 659 (2001)
V. Teske, E. Vogel, Fluid Phase Equilib. 303, 126 (2011)
E. Vogel, K. Dobbert, K. Meissner, U. Ruh, E. Bich, Int. J. Thermophys. 12, 469 (1991)
E. Vogel, Fluid Phase Equilib. 88, 277 (1993)
M.J. Assael, E. Bich, E. Vogel, J. Non-Equilib. Thermodyn. 19, 47 (1994)
E. Vogel, Int. J. Thermophys. 37, 63 (2016)
E. Vogel, R. Span, S. Herrmann, J. Phys. Chem. Ref. Data 44, 043101 (2015)
E. Vogel, S. Herrmann, J. Phys. Chem. Ref. Data 45, 043103 (2016)
S. Herrmann, E. Vogel, J. Phys. Chem. Ref. Data 47, 043103 (2018)
E. Vogel, Wiss. Z. Univ. Rostock Math.-Nat. Reihe 21(2), 169 (1972)
E. Vogel, E. Bastubbe, S. Rohde, Wiss. Z. W.-Pieck-Univ. Rostock Naturwiss. Reihe 33(8), 34 (1984)
J.H. Whitelaw, J. Sci. Instrum. 41, 215 (1964)
T. Strehlow, E. Vogel, E. Bich, Wiss. Z. W.-Pieck-Univ. Rostock Naturwiss. Reihe 35(7), 5 (1986)
G.F. Newell, Z. Angew, Math. Phys. 10, 160 (1959)
H. Iwasaki, J. Kestin, Physica 29, 1345 (1963)
E. Vogel, Int. J. Thermophys. 31, 447 (2010)
E. Vogel, J. Chem. Eng. Data 56, 3265 (2011)
E. Vogel, Int. J. Thermophys. 33, 741 (2012)
E. Vogel, E. Bich, Z. Phys. Chem. 227, 315 (2013)
R. Hellmann, Private Communication (Helmut Schmidt University/University of the Bundeswehr, Hamburg, 2020)
W. Cencek, M. Przybytek, J. Komasa, J.B. Mehl, B. Jeziorski, K. Szalewicz, J. Chem. Phys. 136, 224303 (2012)
H. Preston-Thomas, Metrologia 27, 3 (1990)
E.W. Lemmon, I.H. Bell, M.L. Huber, M.O. McLinden, NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties—REFPROP, Version 10.0 (Standard Reference Data Program, National Institute of Standards and Technology, Gaithersburg, 2018)
J. Gmehling, J. Chem. Eng. Data 27, 371 (1982)
B. Nienhaus, U. Limbeck, R. Bölts, A.B. de Haan, S.H. Niemann, J. Gmehling, J. Chem. Eng. Data 43, 941 (1998)
M. Jaeschke, Int. J. Thermophys. 8, 81 (1987)
B.A. Younglove, J.F. Ely, J. Phys. Chem. Ref. Data 16, 577 (1987)
C. Tsonopoulos, Adv. Chem. Ser. 182, 143 (1979)
S. Hendl, A.K. Neumann, E. Vogel, High Temp.-High Press. 25, 503 (1993)
S. Hendl, E. Vogel, Fluid Phase Equilib. 76, 259 (1992)
E. Vogel, Int. J. Thermophys. 16, 1335 (1995)
C. Küchenmeister, E. Vogel, Int. J. Thermophys. 21, 329 (2000)
E. Vogel, E. Bich, R. Nimz, Physica A 139, 188 (1986)
E. Vogel, B. Holdt, T. Strehlow, Physica A 148, 46 (1988)
E. Bich, G. Opel, R. Pietsch, E. Vogel, Z. Phys. Chem. Leipzig 260, 1145 (1979)
E. Bich, G. Opel, R. Pietsch, R. Schmal, E. Vogel, Z. Phys. Chem. Leipzig 265, 101 (1984)
V. Teske, E. Vogel, J. Chem. Eng. Data 51, 628 (2006)
V. Teske, D. Buttig, E. Vogel, Fluid Phase Equilib. 293, 190 (2010)
E. Vogel, S. Hendl, Fluid Phase Equilib. 79, 313 (1992)
E. Vogel, G. Opel, W. Lichtenstein, Z. Phys. Chem. Leipzig 254, 113 (1973)
E. Vogel, G. Opel, Z. Phys. Chem. Leipzig 263, 753 (1982)
E. Bich, K. Fiedler, G. Opel, E. Vogel, Z. Phys. Chem. Leipzig 262, 402 (1981)
E. Vogel, A.K. Neumann, Int. J. Thermophys. 14, 805 (1993)
G. Opel, U. Stechow, E. Vogel, Z. Phys. Chem. Leipzig 259, 944 (1978)
D.G. Friend, J.C. Rainwater, Chem. Phys. Lett. 107, 590 (1984)
J.C. Rainwater, D.G. Friend, Phys. Rev. A 36, 4062 (1987)
E. Bich, E. Vogel, Int. J. Thermophys. 12, 27 (1991)
E. Bich, E. Vogel, Dense Fluids. Initial Density Dependence (Cambridge University Press, Cambridge, 1996), pp. 72–82
E. Vogel, C. Küchenmeister, E. Bich, A. Laesecke, J. Phys. Chem. Ref. Data 27, 947 (1998)
G.C. Maitland, M. Rigby, E.B. Smith, W.A. Wakeham, Intermolecular Forces: Their Origin and Determination (Clarendon Press, Oxford, 1987)
E. Bich, J. Millat, E. Vogel, Wiss. Z. W.-Pieck-Univ. Rostock Naturwiss. Reihe 36(8), 5 (1987)
J.L. Copp, T.J.V. Findlay, Trans. Faraday Soc. 56, 13 (1960)
K.W. Chun, R.R. Davison, J. Chem. Eng. Data 17, 307 (1972)
R. Hellmann, Chem. Phys. Lett. 613, 133 (2014)
R. Hellmann, J. Chem. Eng. Data 63, 470 (2018)
R. Hellmann, J. Chem. Eng. Data 65, 968 (2020)
R. Hellmann, Private Communication (Helmut Schmidt University/University of the Bundeswehr, Hamburg, 2022)
F.R.W. McCourt, J.J.M. Beenakker, W.E. Köhler, I. Kuščer, Nonequilibrium Phenomena in Polyatomic Gases, Vol. I: Dilute Gases (Clarendon Press, Oxford, 1990)
A.S. Dickinson, R. Hellmann, E. Bich, E. Vogel, Phys. Chem. Chem. Phys. 9, 2836 (2007)
E. Bich, J.B. Mehl, R. Hellmann, V. Vesovic, Experimental Thermodynamics Volume IX: Advances in Transport Properties of Fluids (The Royal Society of Chemistry, Cambridge, 2014), pp. 226–252
R. Di Pippo, J.R. Dorfman, J. Kestin, H.E. Khalifa, Physica A 86, 205 (1977)
J. Kestin, O. Korfali, J.V. Sengers, R. Kamgar-Parsi, Physica A 106, 415 (1981)
V. Vesovic, W.A. Wakeham, Int. J. Thermophys. 10, 125 (1989)
V. Vesovic, W.A. Wakeham, Chem. Eng. Sci. 44, 2181 (1989)
S. Hendl, E. Vogel, Int. J. Thermophys. 16, 1245 (1995)
W.A. Wakeham, V. Vesovic, E. Vogel, S. Hendl, Binary Mixtures: Carbon Dioxide–Ethane (Cambridge University Press, Cambridge, 1996), pp. 288–399
K. Humberg, M. Richter, J.P.M. Trusler, R. Span, J. Chem. Thermodyn. 120, 191 (2018)
K. Humberg, M. Richter, J.P.M. Trusler, R. Span, J. Chem. Thermodyn. 147, 106104 (2020)
J.H. Dymond, K.N. Marsh, R.C. Wilhoit, K.C. Wong, Virial Coefficients of Pure Gases, vol. 21A (Springer, Berlin, 2002), pp. 1–309
Y. Abe, J. Kestin, H.E. Khalifa, W.A. Wakeham, Ber. Bunsenges. Phys. Chem. 54, 1 (1979)
Acknowledgments
Particular thanks to Dr. Robert Hellmann (Helmut-Schmidt-Universität/Universität der Bundeswehr Hamburg, Germany) for his great job to calculate the different transport properties at the respective temperatures of this paper and for his stimulating discussion of this manuscript.
Funding
There was not any funding for this research.
Author information
Authors and Affiliations
Contributions
EV has prepared the manuscript alone.
Corresponding author
Ethics declarations
Ethical Approval
Not Applicable.
Conflict of interest
There exist no conflict interests of a financial or personal nature.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vogel, E. Viscosity for Eight Gaseous and Vapor Mixtures: Revisited from Experiment Between 297 K and 638 K. Final and Preliminary Values for the Interaction Viscosity and for the Product of Molar Density and Diffusion Coefficient in the Limit of Zero Density. Int J Thermophys 44, 75 (2023). https://doi.org/10.1007/s10765-023-03174-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-023-03174-6