Abstract
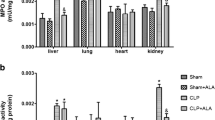
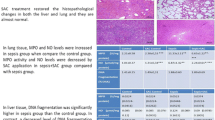
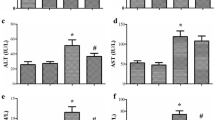
Sepsis is defined as life-threatening organ dysfunction induced by a disrupted host response to infecting pathogens. Evidences suggest that oxidative stress is intrinsically related to sepsis progression. Dimethyl fumarate (DMF) is a novel oral therapeutic agent with anti-oxidant properties which exerts protective effects through activation of nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2). Thus, the aim of this study is to evaluate the effect of DMF in different organs of rats submitted to an animal model of sepsis. Adult male Wistar rats were subjected to sepsis by cecal ligation and puncture (CLP) procedure and sham-operated rats was considered control group. The experimental groups were divided into sham + vehicle, sham + DMF, sham + NAC, CLP + vehicle, CLP + DMF, and CLP + NAC. Rats were treated by oral gavage with DMF immediately after and 12 h after surgery, or NAC (s.c.) at 3, 6, and 12 h after surgery. Twenty-four hours after sepsis induction, neutrophil infiltration, nitrite/nitrate concentrations, oxidative damage to lipids and proteins, superoxide dismutase (SOD), and catalase (CAT) activities were evaluated in the heart, liver, lung, and kidney. Septic animals presented increased neutrophil infiltration, NO metabolism, oxidative damage to lipids and proteins, and decreases of SOD and CAT activities, mainly in the heart, liver, and lung, while DMF-treated animals showed significant reduction in neutrophil infiltration, NO metabolism, and oxidative damage followed by increased SOD and CAT activities. DMF is effective in preventing oxidative stress and inflammation in rats 24 h after sepsis induction.






Similar content being viewed by others
References
Singer, Mervyn, D.S. Deutschman, C.W. Seymour, M. Shankar-Hari, D. Annane, M. Bauer, R. Bellomo, and G.R. Bernard. 2016. The third international consensus definitions for sepsis and septic shock (sepsis-3). The Journal of the American Medical Association 315: 801–810. https://doi.org/10.1001/jama.2016.0287.The.
Van der Poll, Tom. 2016. Future of sepsis therapies. Critical Care 20: 106. https://doi.org/10.1186/s13054-016-1274-9.
Morin, Emily E., L. Guo, A. Schwendeman, and X.A. Li. 2015. HDL in sepsis—risk factor and therapeutic approach. Frontiers in Pharmacology 6: 1–9. https://doi.org/10.3389/fphar.2015.00244.
Petronilho, Fabricia, D. Florentino, L.G. Danielski, L.C. Vieira, M.M. Martins, A. Vieira, S. Bonfante, M.P. Goldim, and F. Vuolo. 2015. Alpha-lipoic acid attenuates oxidative damage in organs after sepsis. Inflammation 39: 357–365. https://doi.org/10.1007/s10753-015-0256-4.
Polat, Gizem, R.A. Ugan, E. Cadirci, and Z. Halici. 2017. Sepsis and septic shock: current treatment strategies and new approaches. The Eurasian Journal of Medicine 49: 53–58. https://doi.org/10.5152/eurasianjmed.2017.17062.
Bone, R.C. 1991. Gram-negative sepsis. Background, clinical features, and intervention. Chest 100: 802–808.
Hattori, Yuichi, K. Hattori, T. Suzuki, and N. Matsuda. 2017. Recent advances in the pathophysiology and molecular basis of sepsis-associated organ dysfunction: novel therapeutic implications and challenges. Pharmacology & Therapeutics. https://doi.org/10.1016/j.pharmthera.2017.02.040.
Andrades, Michael Everton, C. Ritter, and F. Dal-Pizzol. 2009. The role of free radicals in sepsis development. Frontiers in Bioscience 1: 277–287.
Barichello, Tatiana, J.J. Fortunato, A.M. Vitali, G. Feier, A. Reinke, J.C.F. Moreira, J. Quevedo, and F. Dal-Pizzol. 2006. Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Critical Care Medicine 34: 886–889. https://doi.org/10.1097/01.CCM.0000201880.50116.12.
Victor, Victor M., M. Rocha, J.V. Esplugues, and M. De la Fuente. 2005. Role of free radicals in sepsis: antioxidant therapy. Current Pharmaceutical Design 11: 3141–3158.
Hotchkiss, Richard S., and I.E. Karl. 2003. The pathophysiology and treatment of sepsis. The New England Journal of Medicine 348: 138–150. https://doi.org/10.1056/NEJMra021333.
Ritter, Cristiane, M. Andrades, M.L.C. Frota, F. Bonatto, R.A. Pinho, M. Polydoro, F. Klamt, et al. 2003. Oxidative parameters and mortality in sepsis induced by cecal ligation and perforation. Intensive Care Medicine 29: 1782–1789. https://doi.org/10.1007/s00134-003-1789-9.
Andrades, Michael, C. Ritter, J.C.F. Moreira, and F. Dal-Pizzol. 2005. Oxidative parameters differences during non-lethal and lethal sepsis development. Journal of Surgical Research 125: 68–72. https://doi.org/10.1016/j.jss.2004.11.008.
Andrades, Michael, C. Ritter, M.R. De Oliveira, E.L. Streck, J.C. Fonseca Moreira, and F. Dal-Pizzol. 2011. Antioxidant treatment reverses organ failure in rat model of sepsis: role of antioxidant enzymes imbalance, neutrophil infiltration, and oxidative stress. Journal of Surgical Research 167: e307–e313. https://doi.org/10.1016/j.jss.2009.08.005.
Ritter, Cristiane, M.E. Andrades, A. Reinke, S. Menna-Barreto, J.C.F. Moreira, and F. Dal-Pizzol. 2004. Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Critical Care Medicine 32: 342–349. https://doi.org/10.1097/01.CCM.0000109454.13145.CA.
Albrecht, Philipp, I. Bouchachia, C. Zimmermann, H.H. Hofstetter, Z. Kovacs, N. Henke, D. Lisak, et al. 2012. Effects of dimethyl fumarate on neuroprotection and immunomodulation. Journal of Neuroinflammation 9: 163. https://doi.org/10.1186/1742-2094-9-163.
Parodi, Benedetta, C. Cordano, S. Morando, A. Bragoni, D. Giunti, C. Usai, D. Centonze, N.K. De Rosbo, R.H. Scannevin, and A. Uccelli. 2014. Monomethyl fumarate inhibits the NFkB pathway and pro-inflammatory cytokine expression in microglia through HCA2 signaling via the AMPK/Sirt axis. Journal of Neuroimmunology 275: 167–168. https://doi.org/10.1016/j.jneuroim.2014.08.450.
Werdenberg, D., R. Joshi, S. Wolffram, H.P. Merkle, and P. Langguth. 2003. Presystemic metabolism and intestinal absorption of antipsoriatic fumaric acid esters. Biopharmaceutics & Drug Disposition 24: 259–273. https://doi.org/10.1002/bdd.364.
Litjens, N.H.R., J. Burggraaf, E. Van Strijen, C. Van Gulpen, H. Mattie, R.C. Schoemaker, J.T. Van Dissel, H.B. Thio, and P.H. Nibbering. 2004. Pharmacokinetics of oral fumarates in healthy subjects. British Journal of Clinical Pharmacology 58: 429–432. https://doi.org/10.1111/j.1365-2125.2004.02145.x.
Ma, Qiang. 2013. Role of Nrf2 in oxidative stress and toxicity. Annual Review of Pharmacology and Toxicology 53: 401–426. https://doi.org/10.1146/annurev-pharmtox-011112-140320.
Linker, R.A., D.H. Lee, S. Ryan, A.M. Van Dam, R. Conrad, P. Bista, W. Zeng, et al. 2011. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 134: 678–692. https://doi.org/10.1093/brain/awq386.
Jing, X., H. Shi, C. Zhang, M. Ren, M. Han, X. Wei, X. Zhang, and H. Lou. 2015. Dimethyl fumarate attenuates 6-OHDA-induced neurotoxicity in SH-SY5Y cells and in animal model of Parkinson’s disease by enhancing Nrf2 activity. Neuroscience 286: 131–140. https://doi.org/10.1016/j.neuroscience.2014.11.047.
Wilms, Henrik, J. Sievers, U. Rickert, M. Rostami-Yazdi, U. Mrowietz, and R. Lucius. 2010. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammation. Journal of Neuroinflammation 7: 30. https://doi.org/10.1186/1742-2094-7-30.
Scannevin, R.H., S. Chollate, M.-Y. Jung, M. Shackett, H. Patel, P. Bista, W. Zeng, et al. 2012. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. Journal of Pharmacology and Experimental Therapeutics 341: 274–284. https://doi.org/10.1124/jpet.111.190132.
Wierinckx, Anne, J. Brevé, D. Mercier, M. Schultzberg, B. Drukarch, and A.M. Van Dam. 2005. Detoxication enzyme inducers modify cytokine production in rat mixed glial cells. Journal of Neuroimmunology 166: 132–143. https://doi.org/10.1016/j.jneuroim.2005.05.013.
Fink, M.P., and S.O. Heard. 1990. Laboratory models of sepsis and septic shock. The Journal of Surgical Research 49: 186–196.
Carvalho, Dickson, F. Petronilho, F. Vuolo, R.A. Machado, L. Constantino, R. Guerrini, G. Calo, E.C. Gavioli, E.L. Streck, and F. Dal-Pizzol. 2008. The nociceptin/orphanin FQ-NOP receptor antagonist effects on an animal model of sepsis. Intensive Care Medicine 34: 2284–2290. https://doi.org/10.1007/s00134-008-1313-3.
Reick, Christiane, G. Ellrichmann, J. Thone, R.H. Scannevin, C. Saft, R.A. Linker, and R. Gold. 2014. Neuroprotective dimethyl fumarate synergizes with immunomodulatory interferon beta to provide enhanced axon protection in autoimmune neuroinflammation. Experimental Neurology 257: 50–56. https://doi.org/10.1016/j.expneurol.2014.04.003.
Kramer, Tobias, Theresa Grob, Lutz Menzel, Tobias Hirnet, Eva Griemert, Konstantin Radyushkin, Serge C. Thal, Axel Methner, and Michael K.E. Schaefer. 2017. Dimethyl fumarate treatment after traumatic brain injury prevents depletion of antioxidative brain glutathione and confers neuroprotection. Journal of Neurochemistry. https://doi.org/10.1111/jnc.14220.
Barichello, Tatiana, R.A. Machado, L. Constantino, S.S. Valvassori, G.Z. Réus, M.R. Martins, F. Petronilho, C. Ritter, J. Quevedo, and F. Dal-Pizzol. 2007. Antioxidant treatment prevented late memory impairment in an animal model of sepsis. Critical Care Medicine 35: 2186–2190. https://doi.org/10.1097/01.CCM.0000281452.60683.96.
Borgstrom, L., B. Kagedal, and O. Paulsen. 1986. Pharmacokinetics of N-acetylcysteine in man. European Journal of Clinical Pharmacology 31: 217–222.
De Young, L.M., J.B. Kheifets, S.J. Ballaron, and J.M. Young. 1989. Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents and Actions 26: 335–341.
Green, L.C., D.A. Wagner, J. Glogowski, P.L. Skipper, J.S. Wishnok, and S.R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Analytical Biochemistry 126: 131–138. https://doi.org/10.1016/0003-2697(82)90118-X.
Draper, H.H., and M. Hadley. 1990. Malondialdehyde determination as index of lipid peroxidation. Methods in Enzymology 186: 421–431.
Levine, R.L., D. Garland, C.N. Oliver, A. Amici, I. Climent, A.G. Lenz, B.W. Ahn, S. Shaltiel, and E.R. Stadtman. 1990. Determination of carbonyl content in oxidatively modified proteins. Methods in Enzymology 186: 464–478.
Bannister, J.V., and L. Calabrese. 1987. Assays for superoxide dismutase. Methods of Biochemical Analysis 32: 279–312.
Aebi, H. 1984. Catalase in vitro. Methods in Enzymology 105: 121–126.
Lowry, O.H., N.J. Rosebrough, A.L. Farr, and R.J. Randall. 1951. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry 193: 265–275.
Li, Cheng Chung, H.T. Yang, Y.C. Hou, Y.S. Chiu, and W.C. Chiu. 2014. Dietary fish oil reduces systemic inflammation and ameliorates sepsis-induced liver injury by up-regulating the peroxisome proliferator-activated receptor gamma-mediated pathway in septic mice. Journal of Nutritional Biochemistry 25: 19–25. https://doi.org/10.1016/j.jnutbio.2013.08.010.
Papayannopoulos, Venizelos, Kathleen D. Metzler, Abdul Hakkim, and Arturo Zychlinsky. 2010. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. The Journal of Cell Biology 191: 677–691. https://doi.org/10.1083/jcb.201006052.
Mishra, Hemant K., J. Ma, and B. Walcheck. 2017. Ectodomain shedding by ADAM17: its role in neutrophil recruitment and the impairment of this process during sepsis. Frontiers in Cellular and Infection Microbiology 7: 138. https://doi.org/10.3389/fcimb.2017.00138.
Shen, Xiao-Fei, K. Cao, J.-P. Jiang, W.-X. Guan, and J.-F. Du. 2017. Neutrophil dysregulation during sepsis: an overview and update. Journal of Cellular and Molecular Medicine. https://doi.org/10.1111/jcmm.13112.
Lerman, Yelena V., and M. Kim. 2015. Neutrophil migration under normal and sepsis conditions. Cardiovascular & Hematological Disorders Drug Targets 15: 19–28.
Müller, Susen, M. Behnen, K. Bieber, S. Möller, L. Hellberg, M. Witte, M. Hänsel, et al. 2016. Dimethylfumarate impairs neutrophil functions. Journal of Investigative Dermatology 136: 117–126. https://doi.org/10.1038/JID.2015.361.
Chen, Hui, J.C. Assmann, A. Krenz, M. Rahman, M. Grimm, C.M. Karsten, J. Kohl, S. Offermanns, N. Wettschureck, and M. Schwaninger. 2014. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. The Journal of Clinical Investigation 124: 2188–2192. https://doi.org/10.1172/JCI72151.
Liu, Xiuting, W. Zhou, X. Zhang, P. Lu, Q. Du, L. Tao, Y. Ding, Y. Wang, and R. Hu. 2016. Dimethyl fumarate ameliorates dextran sulfate sodium-induced murine experimental colitis by activating Nrf2 and suppressing NLRP3 inflammasome activation. Biochemical Pharmacology 112: 37–49. https://doi.org/10.1016/j.bcp.2016.05.002.
Jobgen, Wenjuan S., S.C. Jobgen, H. Li, C.J. Meininger, and G. Wu. 2007. Analysis of nitrite and nitrate in biological samples using high-performance liquid chromatography. Journal of Chromatography B 851: 71–82. https://doi.org/10.1016/j.jchromb.2006.07.018.
Pfeilschifter, Josef, W. Eberhardt, and A. Huwiler. 2003. Nitric oxide and mechanisms of redox signaling. Journal of the American Society of Nephrology 14: S237–S240.
Stamler, Jonathan S. 1994. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell 78: 931–936. https://doi.org/10.1016/0092-8674(94)90269-0.
Lobo, Suzana M, Francisco G Soriano, Denise F Barbeiro, Daniel De Backer, Qinghua Sun, Zizhi Tu, George Dimopoulos, et al. 2009. Effects of dobutamine on gut mucosal nitric oxide production during endotoxic shock in rabbits. Medical Science Monitor 15. United States: BR37–42.
Soriano, Francisco Garcia, C.B. Lorigados, P. Pacher, and C. Szabo. 2011. Effects of a potent peroxynitrite decomposition catalyst in murine models of endotoxemia and sepsis. Shock 35: 560–566. https://doi.org/10.1097/SHK.0b013e31820fe5d5.
Lin, Shao Xia, L Lisi, C Dello Russo, P E Polak, A Sharp, G Weinberg, S Kalinin, and D L Feinstein. 2011. The anti-inflammatory effects of dimethyl fumarate in astrocytes involve glutathione and haem oxygenase-1. ASN Neuro 3. United States. https://doi.org/10.1042/AN20100033.
Victor, Victor M., J.V. Espulgues, A. Hernandez-Mijares, and M. Rocha. 2009. Oxidative stress and mitochondrial dysfunction in sepsis: a potential therapy with mitochondria-targeted antioxidants. Infectious Disorders Drug Targets 9: 376–389.
Prauchner, Carlos André. 2017. Oxidative stress in sepsis: pathophysiological implications justifying antioxidant co-therapy. Burns 43: 471–485. https://doi.org/10.1016/j.burns.2016.09.023.
Esteban-Zubero, Eduardo, M.A. Alatorre-Jimenez, L. Lopez-Pingarron, M.C. Reyes-Gonzales, P. Almeida-Souza, A. Cantin-Golet, F.J. Ruiz-Ruiz, D.-X. Tan, J.J. Garcia, and R.J. Reiter. 2016. Melatonin’s role in preventing toxin-related and sepsis-mediated hepatic damage: a review. Pharmacological Research 105: 108–120. https://doi.org/10.1016/j.phrs.2016.01.018.
Makled, Mirhan N., M.S. El-Awady, R.R. Abdelaziz, N. Atwan, E.T. Guns, N.M. Gameil, A.B. Shehab El-Din, and E.M. Ammar. 2016. Pomegranate protects liver against cecal ligation and puncture-induced oxidative stress and inflammation in rats through TLR4/NF-kappaB pathway inhibition. Environmental Toxicology and Pharmacology 43: 182–192. https://doi.org/10.1016/j.etap.2016.03.011.
Xiao, Xuefei, M. Yang, D. Sun, and S. Sun. 2012. Curcumin protects against sepsis-induced acute lung injury in rats. Journal of Surgical Research 176: e31–e39. https://doi.org/10.1016/j.jss.2011.11.1032.
Liu, Chun-Hong, W.-D. Zhang, J.-J. Wang, and S.-D. Feng. 2016. Senegenin ameliorate acute lung injury through reduction of oxidative stress and inhibition of inflammation in cecal ligation and puncture-induced sepsis rats. Inflammation 39: 900–906. https://doi.org/10.1007/s10753-016-0322-6.
Aydin, Sevtap, T.T. Sahin, M. Bacanli, G. Taner, A.A. Basaran, M. Aydin, and N. Basaran. 2016. Resveratrol protects sepsis-induced oxidative DNA damage in liver and kidney of rats. Balkan Medical Journal 33: 594–601. https://doi.org/10.5152/balkanmedj.2016.15516.
Taner, Gokce, S. Aydin, M. Bacanli, Z. Sarigol, T. Sahin, A.A. Basaran, and N. Basaran. 2014. Modulating effects of pycnogenol(R) on oxidative stress and DNA damage induced by sepsis in rats. Phytotherapy Research: PTR 28: 1692–1700. https://doi.org/10.1002/ptr.5184.
Mittal, Manish, M.R. Siddiqui, K. Tran, S.P. Reddy, and A.B. Malik. 2014. Reactive oxygen species in inflammation and tissue injury. Antioxidants & Redox Signaling 20: 1126–1167. https://doi.org/10.1089/ars.2012.5149.
Sies, Helmut. 2014. Role of metabolic H2O2 generation: redox signaling and oxidative stress. The Journal of Biological Chemistry 289: 8735–8741. https://doi.org/10.1074/jbc.R113.544635.
Nguyen, Truyen, P. Nioi, and C.B. Pickett. 2009. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. The Journal of Biological Chemistry 284: 13291–13295. https://doi.org/10.1074/jbc.R900010200.
Brennan, Melanie S., M.F. Matos, K.E. Richter, B. Li, and R.H. Scannevin. 2017. The NRF2 transcriptional target, OSGIN1, contributes to monomethyl fumarate-mediated cytoprotection in human astrocytes. Scientific Reports 7: 42054. https://doi.org/10.1038/srep42054.
Miao, Weimin, L Hu, P J Scrivens, and G Batist. 2005. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. The Journal of Biological Chemistry 280. United States: 20340–20348. https://doi.org/10.1074/jbc.M412081200.
Zhao, Xiurong, G. Sun, J. Zhang, S.-M. Ting, N. Gonzales, and J. Aronowski. 2015. Dimethyl fumarate protects brain from damage produced by intracerebral hemorrhage by mechanism involving Nrf2. Stroke 46: 1923–1928. https://doi.org/10.1161/STROKEAHA.115.009398.
Ishii, T., K. Itoh, S. Takahashi, H. Sato, T. Yanagawa, Y. Katoh, S. Bannai, and M. Yamamoto. 2000. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. The Journal of Biological Chemistry 275: 16023–16029.
Shen, Guoxiang, C. Xu, R. Hu, M.R. Jain, A. Gopalkrishnan, S. Nair, M.-T. Huang, J.Y. Chan, and A.-N.T. Kong. 2006. Modulation of nuclear factor E2-related factor 2-mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Molecular Cancer Therapeutics 5: 39–51. https://doi.org/10.1158/1535-7163.MCT-05-0293.
Pan, Hao, H. Wang, X. Wang, L. Zhu, and L. Mao. 2012. The absence of Nrf2 enhances NF-kappaB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediators of Inflammation 2012: 217580. https://doi.org/10.1155/2012/217580.
Iizuka, Takashi, Y. Ishii, K. Itoh, T. Kiwamoto, T. Kimura, Y. Matsuno, Y. Morishima, et al. 2005. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes to Cells: Devoted to Molecular & Cellular Mechanisms 10: 1113–1125. https://doi.org/10.1111/j.1365-2443.2005.00905.x.
Jin, Wei, H. Wang, Y. Ji, Q. Hu, W. Yan, G. Chen, and H. Yin. 2008. Increased intestinal inflammatory response and gut barrier dysfunction in Nrf2-deficient mice after traumatic brain injury. Cytokine 44: 135–140. https://doi.org/10.1016/j.cyto.2008.07.005.
Jin, Wei, L. Zhu, Q. Guan, G. Chen, Q.F. Wang, H. Xia Yin, C.H. Hang, J.X. Shi, and H.D. Wang. 2008. Influence of Nrf2 genotype on pulmonary NF-kappaB activity and inflammatory response after traumatic brain injury. Annals of Clinical and Laboratory Science 38: 221–227.
Selvaraj, Vellaisamy, N. Nepal, S. Rogers, N.D.P.K. Manne, R. Arvapalli, K.M. Rice, S. Asano, et al. 2015. Inhibition of MAP kinase/NF-kB mediated signaling and attenuation of lipopolysaccharide induced severe sepsis by cerium oxide nanoparticles. Biomaterials 59: 160–171. https://doi.org/10.1016/j.biomaterials.2015.04.025.
Lolis, Elias, and Richard Bucala. 2003. Therapeutic approaches to innate immunity: Severe sepsis and septic shock. Nature Reviews. Drug Discovery 2: 635–645. https://doi.org/10.1038/nrd1153.
Spasojevic, Ivan, B. Obradovic, and S. Spasic. 2012. Bench-to-bedside review: neonatal sepsis-redox processes in pathogenesis. Critical Care 16: 221. https://doi.org/10.1186/cc11183.
Loewe, Robert, W. Holnthoner, M. Groger, M. Pillinger, F. Gruber, D. Mechtcheriakova, E. Hofer, K. Wolff, and P. Petzelbauer. 2002. Dimethylfumarate inhibits TNF-induced nuclear entry of NF-kappa B/p65 in human endothelial cells. Journal of Immunology 168: 4781–4787.
Seidel, P., I. Merfort, J.M. Hughes, B.G.G. Oliver, M. Tamm, and M. Roth. 2009. Dimethylfumarate inhibits NF-{kappa}B function at multiple levels to limit airway smooth muscle cell cytokine secretion. American Journal of Physiology. Lung Cellular and Molecular Physiology 297: L326–L339. https://doi.org/10.1152/ajplung.90624.2008.
Schilling, S., S. Goelz, R. Linker, F. Luehder, and R. Gold. 2006. Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clinical and Experimental Immunology 145: 101–107. https://doi.org/10.1111/j.1365-2249.2006.03094.x.
Casili, Giovanna, M. Cordaro, D. Impellizzeri, G. Bruschetta, I. Paterniti, S. Cuzzocrea, and E. Esposito. 2016. Dimethyl fumarate reduces inflammatory responses in experimental colitis. Journal of Crohn’s and Colitis 10: 472–483. https://doi.org/10.1093/ecco-jcc/jjv231.
Han, Ranran, J Xiao, H Zhai, and J Hao. 2016. Dimethyl fumarate attenuates experimental autoimmune neuritis through the nuclear factor erythroid-derived 2-related factor 2/hemoxygenase-1 pathway by altering the balance of M1/M2 macrophages. Journal of Neuroinflammation 13. Journal of Neuroinflammation: 97. https://doi.org/10.1186/s12974-016-0559-x.
Robles, Lourdes, N.D. Vaziri, S. Li, C. Takasu, Y. Masuda, K. Vo, S.H. Farzaneh, M.J. Stamos, and H. Ichii. 2015. Dimethyl fumarate ameliorates acute pancreatitis in rodent. Pancreas 44: 441–447. https://doi.org/10.1097/MPA.0000000000000275.
Meili-Butz, Silvia, T. Niermann, E. Fasler-Kan, V. Barbosa, N. Butz, D. John, M. Brink, P.T. Buser, and C.E. Zaugg. 2008. Dimethyl fumarate, a small molecule drug for psoriasis, inhibits nuclear factor-kappaB and reduces myocardial infarct size in rats. European Journal of Pharmacology 586: 251–258. https://doi.org/10.1016/j.ejphar.2008.02.038.
Acknowledgments
This research was supported by grants from CNPq and CAPES (FP). FP and TB are CNPq research fellows. The funding sources had no involvement in the conduction of the research, preparation of the article, or in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Statement
All studies were performed in compliance with the National Institutes of Health Guidelines and with the approval of the Animal Care and Experimentation Committee of UNISUL (protocol number 13.026.4.03.IV), Brazil.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Giustina, A.D., Bonfante, S., Zarbato, G.F. et al. Dimethyl Fumarate Modulates Oxidative Stress and Inflammation in Organs After Sepsis in Rats. Inflammation 41, 315–327 (2018). https://doi.org/10.1007/s10753-017-0689-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0689-z