Abstract
Objective
This study assessed parameters of free radical damage to biomolecules, mitochondrial superoxide production, superoxide dismutase, and catalase activities and their relationship to sepsis mortality.
Design and setting
Prospective animal study in a university laboratory for experimental.
Subjects
140 male Wistar rats.
Interventions
The animals were randomly divided into three groups: sham-operated (n=20), cecal ligation and perforation resuscitated with normal saline (n=40), and cecal ligation and perforation with normal saline plus antibiotics (n=40).
Measurements and results
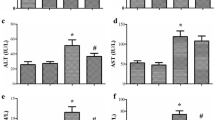
Blood samples were collected from all animals 3, 12, and 24 h after CLP through a jugular catheter inserted before CLP. Rats were evaluated during 5 days after the intervention. Nonsurvivor animals were grouped according to the duration between sepsis induction and death, and oxidative parameters were compared to survivors and sham-operated. Lipid peroxidation, protein carbonyls, and superoxide dismutase were significantly increased in nonsurvivor septic rats and were predictive of mortality. We demonstrated that there is a different modulation of superoxide dismutase and catalase in nonsurvivors during the course of septic response. There was a marked increase in superoxide dismutase activity without a proportional increase in catalase activity in nonsurvivors.
Conclusions
This is the first report of plasma superoxide dismutase as an earlier marker of mortality. Ours results might help to clarify an important aspect of oxidative response to sepsis, i.e., an increase in superoxide dismutase activity without a proportional increase in catalase activity




Similar content being viewed by others
References
Barriere SL, Lowry SF (1995) An overview of mortality risk prediction in sepsis. Crit Care Med 23:376–393
Macarthur H, Westfall TC, Riley DP, Misko TP, Salvemini D (2000) Inactivation of catecholamines by superoxide gives new insights on the pathogenesis of septic shock. Proc Natl Acad Sci USA 97:9753–9758
Salvemini D, Cuzzocrea S (2002) Oxidative stress in septic shock and disseminated intravascular coagulation. Free Radic Biol Med 33:1173–1185
Zimmermann JJ (1995) Defining the role of oxyradicals in the pathogenesis of sepsis. Crit Care Med 23:616–617
Chang CK, Llanes S, Schumer W (1999) Inhibiotry effect of DMSO on nuclear factor-κB activation and intracellular adhesion molecule 1 gene expression in septic rat. J Surg Res 82:294–299
Fujimura N, Sumita S, Aimono M (2000) Effect of free radical scavenger on diaphragmatic contractility in septic peritonitis. Am J Respir Crit Care Med 162:2159–2165
Ortolani O, Conti A, Gaudio AR (2000) The effect of glutathione and N-acetylcysteine on lipoperoxidative damage in patients with early septic shock. Am J Respir Crit Care Med 161:1907–1911
Heller AR, Groth G, Heller SC (2001) N-acetylcysteine reduces respiratory burst but augments neutrophil phagocytosis in intensive care unit patients. Crit Care Med 29:272–276
Rank N, Michel C, Haertel C (2000) NAC increases liver blood flow and improves liver function in septic shock patients: results of a prospective, randomized, double-blind study. Crit Care Med 28:3799–3807
Vulcano M, Meiss RP, Isturiz MA (2000) Deferoxamine reduces tissue injury and lethality in LPS-treated mice. Int J Immunopharmacol 22:635–644
Powell RJ, Machiedo GW, Rush BF Jr, Dikdan GS (1991) Effect of oxygen-free radical scavengers on survival in sepsis. Am Surg 57:86–88
Kunimoto F, Morita T, Ogawa R, Fujita T (1987) Inhibition of lipid peroxidation improves survival rate of endotoxemic rats. Circ Shock 21:15–22
Broner CW, Shenep JL, Stidham GL, Stokes DC, Hildner WK (1988) Effect of scavengers of oxygen-derived free radicals on mortality in endotoxin-challenged mice. Crit Care Med 16:848–851
Kong CW, Tsai K, Chin JH, Chan WL, Hong CY (2000) Magnolol attenuates peroxidative damage and improves survival of rats with sepsis. Shock 13:24–28
Warner BW, Hasselgren PO, James JH (1987) Superoxide dismutase in rats with sepsis. Effect on survival rate and amino acid transport. Arch Surg 122:1142–1146
Broner CW, Shenep JL, Stidham GL (1989) Effect of antioxidants in experimental Escherichia coli septicemia. Circ Shock 29:77–92
Sprong RC, Winkelhuyzen-Janssen AML, Aarsman CJM, van Oirschot JFLM, Bruggen T, van Asbeck BS (1998) Low-dose N-acetylcysteine protects rats against endotoxin-mediated oxidative stress, but high-dose increases mortality. Am J Respir Crit Care Med 157:1283–1293
Wichterman KA, Baue AE, Chaudry IH (1980) Sepsis and septic shock–a review of laboratory models and a proposal. J Surg Res 29:189–199
Hollenberg SM, Dumasius A, Easington C, Colilla SA, Neumann A, Parrillo JE (2001) Characterization of a hyperdynamic murine model of resuscitated sepsis using echocardiography. Am J Respir Crit Care Med 164:891–895
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Levine RL, Garland D, Oliver CN (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Dal-Pizzol F, Klamt F, Bernard EA, Benfato MS, Moreira JCF (2001) Retinol supplementation induces oxidative stress and modulates antioxidant enzyme activities in rat Sertoli cells. Free Radic Res 34:395–404
Bannister JV, Calaberese L (1987) Assays for SOD. Methods Biochem Anal 32:279–312
Poderoso JJ, Carreras MC, Lisdero C, Boveris A (1996) Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondrial and submitochondrial particles. Arch Biochem Biophys 328:85–92
Lloyd SS, Chang AK, Taylor FB Jr (1993) Free radicals and septic shock in primates: the role of tumor necrosis factor. Free Radic Biol Med 14:233–242
Callahan LA, Nethery D, Stofan D, DiMarco A, Supinski G (2001) Free radical-induced contractile protein dysfunction in endotoxin-induced sepsis. Am J Respir Cell Mol Biol 24:210–217
Basu S, Eriksson M (1998) Oxidative injury and survival during endotoxemia. FEBS Lett 438:159–160
Németh I, Boda D (2001) Xanthine oxidase activity and blood glutathione redox ratio in infants and children with septic shock syndrome. Intensive Care Med 27:216–221
Winterbourn CC, Buss IH, Chan TP (2000) Protein carbonyl measurements show evidence of early oxidative stress in critically ill patients. Crit Care Med 28:143–149
McCord JM (1998) The importance of oxidant-antioxidant balance. In: Montagneir L, Olivier R, Pasquier C (eds) Oxidative stress in cancer, AIDS, and neurodegenerative diseases. Dekker, New York, pp 1–8
Klamt F, Dal-Pizzol F, Frota Jr MLC, Andrades M, Silva EG, Walz R, Izquierdo I, Brentani RR, Moreira JCF (2001) Imbalance of antioxidant defences in mice lacking cellular prion protein. Free Radic Biol Med 30:1137–1144
Dal-Pizzol F, Klamt F, Andrades M, Frota Jr MLC, Caregnato F, Quevedo J, Schorder N, Vianna M, Izquierdo I, Archer T, Moreira JCF (2001) Neonatal iron administration induces oxidative stress in adult Wistar rats. Brain Res Dev Brain Res 130:109–114
Dal-Pizzol F, Ritter C, Klamt F, Andrades M, Frota Jr MLC, Diel C, da Rocha A, de Lima C, Braga Filho A, Schwartsmann G, Moreira JCF (2003) Modulation of oxidative stress in response to gamma-radiation in human glioma cell lines. J Neurooncol 61:89–94
Warner A, Bencosme A, Healy D, Verme C (1995) Prognostic role of antioxidant enzymes in sepsis: preliminary assessment. Clin Chem 41:867–871
Bianca REV, Wayman NS, McDonald MC, Pinto A, Sharpe MA, Chatterjee PK, Thiemermann C (2002) Superoxide dismutase mimetic with catalase activity, EUK-134, attenuates the multiple organ injury and dysfunction caused by endotoxin in the rat. Med Sci Monit 8:BR1–BR7
Salvemini D, Cuzzocrea S (2003) Therapeutical potential of superoxide dismutase mimetics as therapeutic agents in critical care medicine. Crit Care Med 31 [Suppl]:29–38
Traber DL, Adams T, Sziebvert L (1985) Potentiation of lung vascular response to endotoxin by superoxide dismutase. J Appl Physiol 58:1005–1009
Olsen NC, Grizzle MK, Anderson DL (1987) Effect of polyethylene glycol-superoxide dismutase and catalase on endotoxemia in pigs. J Appl Physiol 63:1526–1532
Novotny MJ, Laighlin MH, Adams H (1998) Evidence of lack of importance of oxygen free radicals in Escherichia coli endotoxemia in dogs. Am J Physiol 254:H954–H962
McKechnie K, Furman BL, Parrat JR (1986) Modification by oxygen free radical scavengers of the metabolic and cardiovascular effects of endotoxin infusion in conscious rats. Circ Shock 19:429–439
Masuda A, Longo DL, Kobayashi Y (1988) Induction of mitochondrial manganese superoxide dismutase by interleukin 1. FASEB J 2:3087–3091
Visner GA, Douglas WC, Wilson JM, Burr IA, Nick HS (1990) Regulation of manganese superoxide dismutase by lipopolysaccaride, interleukin-1, and tumor necrosis factor. J Biol Chem 265:2856–2864
Gantchev TG, van Lier JE (1995) Catalase inactivation following photosensitization with tetrasulfonated metallophthalocyanines. Photochem Photobiol 62:123–134
Boczkowski J, Lisdero CL, Lanone S, Boveris A, Poderoso JJ (1999) Endogenous peroxynitrite mediates mitochondrial dysfunction in rat diaphragm during endotoxemia. FASEB J 13:1637–1647
Ritter C, Andrades M, Moreira JCF, Dal-Pizzol F (2003) Superoxide production during sepsis development. Am J Respir Crit Care Med 167:474–475
Boveris A, Alvarez S, Navarro A (2002) The role of mitochondrial nitric oxide synthase in inflammation and septic shock. Free Radic Biol Med 33:1186–1193
Sanlioglu S, Williams CM, Samavati L (2001) LPS induces Rac-1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-α secretion through IKK regulation of NF-κB. J Biol Chem 276:30188–30198
Acknowledgements
This work was supported by grants from FAPERGS, FIPE-HCPA, and CNPq. The authors thank to Dr. R. Roesler for his critical revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
An editorial regarding this article can be found in the same issue (http://dx.doi.org/10.1007/s00134-003-1861-5)
Rights and permissions
About this article
Cite this article
Ritter, C., Andrades, M., Frota, M.L.C. et al. Oxidative parameters and mortality in sepsis induced by cecal ligation and perforation. Intensive Care Med 29, 1782–1789 (2003). https://doi.org/10.1007/s00134-003-1789-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-1789-9