Abstract
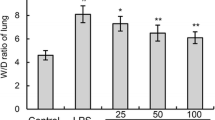
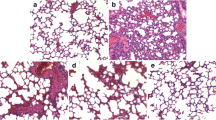
Acute lung injury (ALI) is a life-threatening syndrome which causes a high mortality rate worldwide. In traditional medicine, lots of aromatic plants—such as some Thymus species—are used for treatment of various lung diseases including pertussis, bronchitis, and asthma. Thymol, one of the primary active constituent derived from Thymus vulgaris (thyme), has been reported to exhibit potent anti-microbial, anti-oxidant, and anti-inflammatory activities in vivo and in vitro. The present study aims to investigate the protective effects of thymol in lipopolysaccharide (LPS)-induced lung injury mice model. In LPS-challenged mice, treatment with thymol (100 mg/kg) before or after LPS challenge significantly improved pathological changes in lung tissues. Thymol also inhibited the LPS-induced inflammatory cells influx, TNF-α and IL-6 releases, and protein concentration in bronchoalveolar lavage fluid (BALF). Additionally, thymol markedly inhibited LPS-induced elevation of MDA and MPO levels, as well as reduction of SOD activity. Further study demonstrated that thymol effectively inhibited the NF-κB activation in the lung. Taken together, these results suggested that thymol might be useful in the therapy of acute lung injury.






Similar content being viewed by others
References
Butt, Y., A. Kurdowska, and T.C. Allen. 2016. Acute lung injury: a clinical and molecular review. Archives of Pathology & Laboratory Medicine 140: 345–350.
Blondonnet, R., J.M. Constantin, V. Sapin, and M. Jabaudon. 2016. A pathophysiologic approach to biomarkers in acute respiratory distress syndrome. Disease Markers 2016: 3501373.
Yehya, N., and N.J. Thomas. 2016. Relevant outcomes in pediatric acute respiratory distress syndrome studies. Frontiers in Pediatrics 4: 51.
Papazian, L., C.S. Calfee, D. Chiumello, C.E. Luyt, N.J. Meyer, H. Sekiguchi, M.A. Matthay, and G.U. Meduri. 2016. Diagnostic workup for ARDS patients. Intensive Care Medicine 42: 674–685.
Lee KY. 2017. Pneumonia, acute respiratory distress syndrome, and early immune-modulator therapy. International Journal of Molecular Sciences 18.
Chen, H., C. Bai, and X. Wang. 2010. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Review of Respiratory Medicine 4: 773–783.
Matute-Bello, G., C.W. Frevert, and T.R. Martin. 2008. Animal models of acute lung injury. American Journal of Physiology. Lung Cellular and Molecular Physiology 295: L379–L399.
Zhong, L.L., H.Y. Chen, W.C. Cho, X.M. Meng, and Y. Tong. 2012. The efficacy of Chinese herbal medicine as an adjunctive therapy for colorectal cancer: a systematic review and meta-analysis. Complementary Therapies in Medicine 20: 240–252.
Sun, J., K. Zhang, W.J. Xiong, G.Y. Yang, Y.J. Zhang, C.C. Wang, L. Lai, M. Han, J. Ren, G. Lewith, and J.P. Liu. 2016. Clinical effects of a standardized Chinese herbal remedy, Qili Qiangxin, as an adjuvant treatment in heart failure: systematic review and meta-analysis. BMC Complementary and Alternative Medicine 16: 201.
Vigo, E., A. Cepeda, O. Gualillo, and R. Perez-Fernandez. 2004. In-vitro anti-inflammatory effect of Eucalyptus globulus and Thymus vulgaris: nitric oxide inhibition in J774A.1 murine macrophages. The Journal of Pharmacy and Pharmacology 56: 257–263.
Marti, D., V. Villagrasa, I. Martinez-Solis, A. Blanquer, E. Castillo, and L.M. Royo. 2005. Hystological and pharmacological study of Thymus piperella (L.). Phytotherapy Research 19: 298–302.
Boskabady, M.H., M.R. Aslani, and S. Kiani. 2006. Relaxant effect of Thymus vulgaris on guinea-pig tracheal chains and its possible mechanism(s). Phytotherapy Research 20: 28–33.
Villanueva, B.D., I. Angelov, G. Vicente, R.P. Stateva, G.M. Rodriguez, G. Reglero, E. Ibanez, and T. Fornari. 2015. Extraction of thymol from different varieties of thyme plants using green solvents. Journal of the Science of Food and Agriculture 95: 2901–2907.
Wang, L., X. Zhao, C. Zhu, X. Xia, W. Qin, M. Li, T. Wang, S. Chen, Y. Xu, B. Hang, Y. Sun, J. Jiang, L.P. Richard, L. Lei, G. Zhang, and J. Hu. 2017. Thymol kills bacteria, reduces biofilm formation, and protects mice against a fatal infection of Actinobacillus pleuropneumoniae strain L20. Veterinary Microbiology 203: 202–210.
Botelho, M.A., G. Barros, D.B. Queiroz, C.F. Carvalho, J. Gouvea, L. Patrus, M. Bannet, D. Patrus, A. Rego, I. Silva, G. Campus, and I. Araujo-Filho. 2016. Nanotechnology in phytotherapy: antiinflammatory effect of a nanostructured thymol gel from Lippia sidoides in acute periodontitis in rats. Phytotherapy Research 30: 152–159.
Luna, A., R.C. Lema-Alba, J.S. Dambolena, J.A. Zygadlo, M.C. Labaque, and R.H. Marin. 2017. Thymol as natural antioxidant additive for poultry feed: oxidative stability improvement. Poultry Science 96: 3214–3220.
Manukumar, H.M., S. Umesha, and H. Kumar. 2017. Promising biocidal activity of thymol loaded chitosan silver nanoparticles (T-C@AgNPs) as anti-infective agents against perilous pathogens. International Journal of Biological Macromolecules 102: 1257–1265.
Li-Mei, W., T. Jie, W. Shan-He, M. Dong-Mei, and Y. Peng-Jiu. 2016. Anti-inflammatory and anti-oxidative effects of dexpanthenol on lipopolysaccharide induced acute lung injury in mice. Inflammation 39: 1757–1763.
Schingnitz, U., K. Hartmann, C.F. Macmanus, T. Eckle, S. Zug, S.P. Colgan, and H.K. Eltzschig. 2010. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. Journal of Immunology 184: 5271–5279.
Grommes, J., and O. Soehnlein. 2011. Contribution of neutrophils to acute lung injury. Molecular Medicine 17: 293–307.
Bhatia, M., and S. Moochhala. 2004. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. The Journal of Pathology 202: 145–156.
Sawa, T. 2014. The molecular mechanism of acute lung injury caused by Pseudomonas aeruginosa: from bacterial pathogenesis to host response. Journal of Intensive Care Medicine 2: 10.
Herold, S., N.M. Gabrielli, and I. Vadasz. 2013. Novel concepts of acute lung injury and alveolar-capillary barrier dysfunction. American Journal of Physiology. Lung Cellular and Molecular Physiology 305: L665–L681.
Hooper, M., and G. Bernard. 2011. Pharmacogenetic treatment of acute respiratory distress syndrome. Minerva Anestesiologica 77: 624–636.
Zhou, E., Y. Fu, Z. Wei, Y. Yu, X. Zhang, and Z. Yang. 2014. Thymol attenuates allergic airway inflammation in ovalbumin (OVA)-induced mouse asthma. Fitoterapia 96: 131–137.
Nagoor, M.M., G.S. Jagadeesh, and P. Selvaraj. 2015. Thymol attenuates inflammation in isoproterenol induced myocardial infarcted rats by inhibiting the release of lysosomal enzymes and downregulating the expressions of proinflammatory cytokines. European Journal of Pharmacology 754: 153–161.
Wu, H., K. Jiang, N. Yin, X. Ma, G. Zhao, C. Qiu, and G. Deng. 2017. Thymol mitigates lipopolysaccharide-induced endometritis by regulating the TLR4- and ROS-mediated NF-kappaB signaling pathways. Oncotarget 8: 20042–20055.
Liang, D., F. Li, Y. Fu, Y. Cao, X. Song, T. Wang, W. Wang, M. Guo, E. Zhou, D. Li, Z. Yang, and N. Zhang. 2014. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-kappaB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation 37: 214–222.
Zhou, X., Q. Dai, and X. Huang. 2012. Neutrophils in acute lung injury. Front Biosci (Landmark Ed) 17: 2278–2283.
Aboelwafa, H.R., and H.N. Yousef. 2015. The ameliorative effect of thymol against hydrocortisone-induced hepatic oxidative stress injury in adult male rats. Biochemistry and Cell Biology 93: 282–289.
Nagoor, M.M., G.S. Jagadeesh, and P. Selvaraj. 2016. Thymol, a dietary monoterpene phenol abrogates mitochondrial dysfunction in beta-adrenergic agonist induced myocardial infarcted rats by inhibiting oxidative stress. Chemico-Biological Interactions 244: 159–168.
Kim, Y.S., J.W. Hwang, S.H. Kang, E.H. Kim, Y.J. Jeon, J.H. Jeong, H.R. Kim, S.H. Moon, B.T. Jeon, and P.J. Park. 2014. Thymol from Thymus quinquecostatus Celak. protects against tert-butyl hydroperoxide-induced oxidative stress in Chang cells. Journal of Natural Medicines 68: 154–162.
Bedreag, O.H., A.F. Rogobete, M. Sarandan, A.C. Cradigati, M. Papurica, M.C. Dumbuleu, A.M. Chira, O.M. Rosu, and D. Sandesc. 2015. Oxidative stress in severe pulmonary trauma in critical ill patients. Antioxidant therapy in patients with multiple trauma—a review. Anaesthesiology Intensive Therapy 47: 351–359.
Gawel, S., M. Wardas, E. Niedworok, and P. Wardas. 2004. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiadomości Lekarskie 57: 453–455.
Chung, W.H. 2017. Unraveling new functions of superoxide dismutase using yeast model system: beyond its conventional role in superoxide radical scavenging. Journal of Microbiology 55: 409–416.
Ueda, J., M.E. Starr, H. Takahashi, J. Du, L.Y. Chang, J.D. Crapo, B.M. Evers, and H. Saito. 2008. Decreased pulmonary extracellular superoxide dismutase during systemic inflammation. Free Radical Biology & Medicine 45: 897–904.
Schuliga, M. 2015. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules 5: 1266–1283.
Fudala, R., T.C. Allen, A. Krupa, P.T. Cagle, S. Nash, Z. Gryczynski, I. Gryczynski, and A.K. Kurdowska. 2011. Increased levels of nuclear factor kappaB and Fos-related antigen 1 in lung tissues from patients with acute respiratory distress syndrome. Archives of Pathology & Laboratory Medicine 135: 647–654.
Schwartz, M.D., E.E. Moore, F.A. Moore, R. Shenkar, P. Moine, J.B. Haenel, and E. Abraham. 1996. Nuclear factor-kappa B is activated in alveolar macrophages from patients with acute respiratory distress syndrome. Critical Care Medicine 24: 1285–1292.
Lopez, B., T.M. Maisonet, and V.A. Londhe. 2015. Alveolar NF-kappaB signaling regulates endotoxin-induced lung inflammation. Experimental Lung Research 41: 103–114.
Wang, M., T. Liu, D. Wang, Y. Zheng, X. Wang, and J. He. 2011. Therapeutic effects of pyrrolidine dithiocarbamate on acute lung injury in rabbits. Journal of Translational Medicine 9: 61.
Weng, T.I., H.Y. Wu, C.W. Kuo, and S.H. Liu. 2011. Honokiol rescues sepsis-associated acute lung injury and lethality via the inhibition of oxidative stress and inflammation. Intensive Care Medicine 37: 533–541.
Weifeng, Y., L. Li, H. Yujie, L. Weifeng, G. Zhenhui, and H. Wenjie. 2016. Inhibition of acute lung injury by tnfr-fc through regulation of an inflammation-oxidative stress pathway. PLoS One 11: e151672.
Zhu, T., D.X. Wang, W. Zhang, X.Q. Liao, X. Guan, H. Bo, J.Y. Sun, N.W. Huang, J. He, Y.K. Zhang, J. Tong, and C.Y. Li. 2013. Andrographolide protects against LPS-induced acute lung injury by inactivation of NF-kappaB. PLoS One 8: e56407.
Luo, Y., B. Zhang, D.Q. Xu, Y. Liu, M.Q. Dong, P.T. Zhao, and Z.C. Li. 2011. Protective effect of bicyclol on lipopolysaccharide-induced acute lung injury in mice. Pulmonary Pharmacology & Therapeutics 24: 240–246.
Funding
This study was partly supported by grants from Education Bureau of Guangzhou (No. 1201581610), National Natural Science Foundation of China (No. 81402992), and the Open Project of Guangdong Provincial Key Laboratory of New Drug Screening (2016).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All procedures in this study were performed in accordance with the General Recommendation and Provisions of the Chinese Experimental Animals Administration Legislation.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wan, L., Meng, D., Wang, H. et al. Preventive and Therapeutic Effects of Thymol in a Lipopolysaccharide-Induced Acute Lung Injury Mice Model. Inflammation 41, 183–192 (2018). https://doi.org/10.1007/s10753-017-0676-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0676-4