Abstract
Cancer and cardiovascular diseases, including heart failure (HF), are the main causes of death in Western countries. Several anticancer drugs and radiotherapy have adverse effects on the cardiovascular system, promoting left ventricular dysfunction and ultimately HF. Nonetheless, the relationship between cancer and HF is likely not unidirectional. Indeed, cancer and HF share common risk factors, and both have a bidirectional relationship with systemic inflammation, metabolic disturbances, and neurohormonal and immune activation. Few studies have assessed the impact of untreated cancer on the heart. The presence of an active cancer has been associated with elevated cardiac biomarkers, an initial impairment of left ventricular structure and function, autonomic dysfunction, and reduced exercise tolerance. In turn, these conditions might increase the risk of cardiac damage from chemotherapy and radiotherapy. HF drugs such as beta-blockers or inhibitors of the renin–angiotensin–aldosterone system might exert a protective effect on the heart even before the start of cancer therapies. In this review, we recapitulate the evidence of cardiac involvement in cancer patients naïve from chemotherapy and radiotherapy and no history of cardiac disease. We also focus on the perspectives for an early diagnosis and treatment to prevent the progression to cardiac dysfunction and clinical HF, and the potential benefits of cardioactive drugs on cancer progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent advances in cancer therapies have considerably improved the survival rates of many cancers [1], but several anticancer drugs and radiotherapy regimens have detrimental effects on the heart, leading to pericardial and myocardial disease, left ventricular (LV) dysfunction, and ultimately heart failure (HF) [2]. In this perspective, there is a growing interest in the bidirectional relationship between cancer and HF, beyond the possible effects of cancer therapies. Cancer per se might promote HF development, whereas HF could act as a pro-oncogenic condition [3]. Cancer and HF share several risk factors, such as hypertension, diabetes, obesity, and smoking [4,5,6], and the systemic metabolic alterations could link one disease to the other [7, 8]. While there is evidence that patients with HF have a higher incidence of cancer compared to the general population [9], data on the association between cancer alone (i.e., naïve to therapies) and HF are very limited. Until now, research mainly focused on the long-term risk of cardiovascular disease in cancer survivors [10,11,12].
In this review, we discuss the main evidence of subclinical cardiac damage in treatment-naïve cancer patients, and recapitulate the possible mechanisms leading to HF, including inflammation, oxidative stress, and autonomic impairment. We also discuss the potential roles of drug therapies for the prevention of cancer-related cardiotoxicity, and the potential benefits of cardioactive drugs on cancer progression.
Possible mechanisms leading to HF in cancer patients
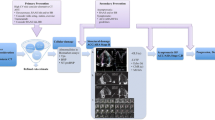
Systemic inflammation and oxidative stress, neurohormonal activation, autonomic dysfunction, clonal haematopoiesis, and metabolic derangements are the main proposed mechanisms whereby cancer may promote cardiac dysfunction, and, ultimately, HF (Fig. 1).
Pathophysiological interplay heart-cancer disease in patients naïve to chemotherapy. Cancer and heart have a bidirectional relationship (cancer to heart; heart to cancer). Besides a direct effect mediated by chemo (and radio) therapy, cancer can impact on heart structure and function in several different pathways: inflammation, oxidative stress, metabolic (oncometabolites), and directly affecting sympathetic nervous system (SNS) activation
Several conditions have detrimental effects on the heart and may lead to HF. Systemic amyloidoses are a group of diseases characterized by the deposition of amyloid, an aggregate of misfolded proteins, in one or more organs. The two most common forms of cardiac amyloidosis are due transthyretin amyloidosis (ATTR) and light-chain amyloidosis (AL), derived from abnormal light chains produced by plasma cell malignancies [13]. Pheochromocytomas, rare catecholamine-secreting tumours of the adrenal glands, often cause a severe cardiotoxicity mediated by β adrenergic receptor stimulation, which manifests in several forms, from Takotsubo to dilated cardiomyopathy [14, 15]. Carcinoid heart disease is the cardiac involvement of serotonin-producing neuroendocrine tumours (NETs), due to fibrotic endocardial plaques with associated valve dysfunction often leading to right-sided HF [16]. In acromegaly, the excess of growth hormone (GH) and insulin-like growth factor 1 (IGF-I) induces a specific cardiomyopathy whose most common feature is concentric hypertrophy, usually associated with diastolic dysfunction and eventual impairment of systolic function and HF [17].
Inflammation and oxidative stress
Most malignancies elicit an inflammatory response through the release of proinflammatory cytokines and acute phase proteins that create a protumorigenic microenvironment, which in turn contributes to cancer invasiveness [18,19,20,21,22,23]. Inflammation also promotes microvascular endothelial dysfunction and HF development (particularly HF with preserved ejection fraction) in experimental models, and proinflammatory cytokines (such as tumour necrosis factor-alpha [TNFα], interleukin-1β and -6) may reduce contractility and promote adverse LV remodelling [3, 24, 25].
Oxidative stress, due for example to reactive oxygen species (ROS), works synergistically with inflammation to promote both cancer and HF [26]. Sustained exposure to ROS can cause DNA damage and further support cancer promotion and progression [27,28,29]. Cancer itself can foster oxidative stress, mainly through the effect of oncogenes (e.g., Myc or Ras) affecting cellular metabolism, redox homeostasis, and DNA replication [30]. Similarly, mitochondrial dysfunction is a prominent feature of HF, resulting in increased cellular levels of ROS and reactive nitrogen species (RNS), altered calcium handling, mitochondrial DNA replication, excitation–contraction coupling, promotion of cardiomyocyte hypertrophy and apoptosis, and myocardial fibrosis [31, 32].
Renin–angiotensin–aldosterone system activation
Increased rennin-angiotensin-aldosterone system (RAAS) activity has been demonstrated in various tumour types, including kidney, prostate, bladder, stomach, cervix, brain, pancreas, colon, lung, liver, skin, and haematopoietic cancers [33, 34]. Angiotensin receptor-1 (ATR1) signalling appears to be the major component of RAAS involved in tumour growth (by inducing angiogenesis) and tumour proliferation (by promoting vascular or epidermal growth factor receptor expression) [33, 35]. ATR1 stimulates inflammation, fibrosis, angiogenesis, tumour invasion and metastasis, while ATR2 antagonize these effects [33, 35]. Angiotensin II can also promote cell growth and proliferation through the transforming growth factor-beta [36], tyrosine kinase [37], and activating mammalian target of rapamycin (mTOR) pathways [38]. Furthermore, a subpopulation of cancer cells known as cancer stem cells (CSCs) has been identified in many types of cancers [39]. These cells express components of the RAAS, supporting the intriguing hypothesis of paracrine mechanisms fostering carcinogenesis via stem cells [40].
The RAAS has a well-established pathogenetic role in HF [41, 42], promoting myocardial hypertrophy and fibrosis and adverse LV remodelling. Thus, enhanced RAAS activity in cancer patients might promote HF development.
Cardiac autonomic dysfunction
The possible occurrence of autonomic dysfunction in cancer is increasingly acknowledged. It is particularly prevalent among patients with advanced cancer and in those undergoing chemotherapy and radiotherapy, but studies suggest an increased prevalence among cancer patients compared to cancer-free individuals. Autonomic dysfunction could be related to preexisting neuropathy, paraneoplastic effects, tumour invasion or compression of autonomic nerves, cancer-related deconditioning, or autoimmune disorders [43,44,45]. Sustained activation of the sympathetic nervous system (SNS) may contribute to cancer initiation [46] and progression [47]. It also influences the tumour microenvironment by promoting the secretion of proinflammatory cytokines [48, 49] and suppressing the immune response [50, 51]. SNS stimulation can also induce cancer cells to escape anoikis, a form of cell death occurring when cells detach from the extracellular matrix [52, 53]. High levels of tissue catecholamines can further promote tumour invasiveness by upregulating matrix metalloproteinases (MMPs) [54, 55], thus increasing vascularisation and matrix degradation, which are the first steps toward metastatic dissemination [24, 56]. Finally, adrenergic stimulation recruits activated macrophages to the tumour parenchyma and induces a prometastatic gene expression signature [57]. These effects are largely mediated by beta-adrenergic receptors, particularly beta-2 adrenoreceptors, and may then be counteracted by beta-blockers [58]. Several preclinical studies show that adrenergic activation modulates apoptosis, promotes angiogenesis and other cancer hallmarks, which provide a rationale for the use of beta-blockers as antineoplastic and cardioprotective agents, and even as adjuvants to cancer chemotherapy [59, 60].
As in the case of RAAS activation, autonomic dysfunction due to cancer might promote the progression to HF. Increased sympathetic outflow and vagal withdrawal is a hallmark of HF [61], particularly HF with reduced ejection fraction (HFrEF), but also mid/range and HFpEF [62]. These changes are initially compensatory, but lead over time to maladaptive ventricular remodelling, hypertrophy, myocardial fibrosis, myocyte cell death, and further deterioration of cardiac function [63].
Clonal haematopoiesis
Clonal haematopoiesis (CH) is a proliferation of haematopoietic stem cells carrying somatic mutations [64]. These mutations occur predominantly in genes encoding for key epigenetic regulators of haematopoiesis [65, 66]. Some of these somatic mutations in the haematopoietic stem cells are linked to an increased risk of coronary artery disease [66, 67]. Other somatic mutations are associated with a poor prognosis in HF patients [68], with their modulation preventing the worsening of cardiac dysfunction in preclinical mouse models [69]. Accordingly, patients with CH have a higher risk of cancer, HF, and death than controls without mutations [70].
Oncometabolic pathways
Oncometabolites are molecules that accumulate in cancer cells, often through mutations of genes encoding the corresponding enzymes, which drive the activation of oncogenic pathways [71]. A preclinical study in rodents showed that an increased production of the oncometabolite D-2-hydroxyglutarate by mutant leukemic cells may reduce cardiac contractility by impairing oxidative decarboxylation of α-ketoglutarate and increasing ATP citrate lyase activity [8]. Similarly, results from genetically engineered and nude mice carrying tumours expressing mutant isocitrate dehydrogenase-2 (IDH2) suggest that D-2-hydroxyglutarate promote the development of cardiomyopathy [72].
An energy shift from mitochondrial oxidative phosphorylation to aerobic glycolysis is often observed in cancer cells. This so-called Warburg effect has been extensively studied in cancer, but there is growing evidence of its involvement even in non-tumour diseases, including HF [73, 74]. A shift from the adult to the foetal isoforms of pyruvate kinase (PKM1 to PKM2) is a hallmark of the Warburg effect, with the latter causing accumulation of intermediates of the glycolytic pathway [75]. A high PKM2 expression has been found not only in cancer cells, but also in the failing hearts of patients with advanced HF, and is partially reversible through mechanical unloading [76].
Evidence of cardiac disease in cancer patients
Characterizing the effects of cancer on the heart requires the assessment of circulating biomarkers, imaging techniques, evaluation of functional capacity and autonomic function (Table 1).
Biomarkers
Laboratory markers can be used to assess a variety of pathophysiological processes in HF, such as fibrosis, inflammation, myocardial injury, and remodelling [77]. There is extensive literature on the role of cardiac biomarkers (particularly natriuretic peptides and high-sensitivity troponins [hs-Tn]) for the early detection of cardiotoxicity from cancer therapies [78,79,80]. Much less is known about circulating levels and prognostic value of biomarkers before the start of chemotherapy.
Recent studies reported elevated levels of several biomarkers of myocardial injury and alterations of immunity and inflammatory pathways in treatment-naïve cancer patients [81,82,83,84,85,86,87,88]. A retrospective study in 145 patients with haematologic and solid organ malignancies not on treatment investigated the levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP). There was evidence of fluid overload in 80% of patients with elevated NT-proBNP. The degree of NT-proBNP elevation was similar between patients with and without HF or volume overload and those with solid vs haematologic malignancies [87]. The role of high-sensitivity troponin (hs-Tn) levels to detect subclinical myocardial damage beyond conventional cardiovascular risk factors has been confirmed in a recent study, including 3512 individuals free from cardiovascular disease [89]. Cancer survivors (19% of the whole population) had significantly higher odds of elevated hs-TnT than patients without prior cancer (odds ratio [OR] 1.26; 95% confidence interval [CI], 1.03–1.53). Furthermore, a significant increase of hs-TnI in 25 haematologic patients naïve from anthracycline therapy has been reported [90]. Overall, despite an association between increased NT-proBNP or hs-Tn, there is limited evidence of a link between this increase and alterations of cardiac structure or function in treatment-naïve cancer patients [91].
A prognostic role of biomarkers in this setting has also been proposed. In a study on 555 patients with a primary diagnosis of cancer and no prior therapies, several biomarkers (including NT-proBNP, hs-TnT, mid-regional proatrial natriuretic peptide, proadrenomedullin, copeptin, and interleukin 6) were shown to increase with tumour progression. All biomarkers predicted mortality regardless of age, gender, tumour entity and stage, and cardiac comorbidities [92]. Recently, hs-TnT before the start of treatment was reported to predict a composite endpoint of cardiovascular death, stroke or transient ischemic attack, pulmonary embolism and new-onset HF, as well as the progression of cardiac involvement at 3 months, in patients on immune checkpoint inhibitors, with 14 ng/L as the best cutoff [88].
In summary, patients with cancer can display an increase in cardiac biomarkers before the start of cancer therapies, and such increase might predict an increased risk of adverse outcome. However, further studies are needed to assess the prognostic role of other cardiac biomarkers, such as soluble suppression of tumorigenicity-2, galectin-3, or fibroblast growth factor-23.
Imaging findings
Echocardiography is the first-line imaging technique to assess cardiac structure and function. Although LV ejection fraction (LVEF) is the most commonly used parameter to detect cardiotoxic damage [79], speckle tracking imaging has emerged as a more sensitive marker of subclinical myocardial dysfunction [79, 93,94,95], with prognostic relevance in terms of prediction of manifest LV dysfunction [96,97,98,99].
Cramer et al. reported increased end-systolic volumes but similar diastolic function parameters in patients with evidence of colorectal cancer (CRC) before chemotherapy [91] compared with controls, but their analysis did not include advanced echocardiographic imaging. A retrospective study on 122 chemotherapy and radiotherapy-naive patients with cancer and 45 controls with similar cardiovascular risk profile showed that cancer, even before the initiation of therapy, was associated with reduced longitudinal (OR 9.0; 95% CI, 2.20–23.50; p < 0.001), circumferential (OR 7.1; 95% CI, 3.80–20.40; p < 0.001), and radial strain (OR 7.2; 95% CI, 3.41–25.10; p < 0.001) regardless of age, sex, body mass index, diabetes, and hypertension [100]. Similarly, cancer was independently associated with reduced right ventricle (RV) global longitudinal strain (OR 3.79; 95% CI, 2.18–10.92; p < 0.001), as well as with decreased free wall RV longitudinal strain (OR 5.73; 95% CI, 3.17–9.85; p < 0.001) [101].
Cardiac magnetic resonance (CMR) allows a characterisation of myocardial tissue changes such as intracellular and interstitial oedema and fibrosis, which may represent early markers of myocardial injury [102]. T1- and T2-weighted imaging and T2 and T1 mapping sequences can help identify intracellular and interstitial oedema [103, 104]. CMR is then a valuable tool for early detection of cardiotoxicity by CMR [105,106,107]. There is currently no evidence of its prognostic value in chemotherapy and radiotherapy-naïve patients.
Exercise capacity
Several studies have reported a significant reduction of functional capacity in patients on chemotherapy or cancer survivors [108,109,110]. The functional implications of cardiorespiratory performance in untreated cancer patients, assessed through cardiopulmonary exercise testing, are less clear. A pilot study evaluated 248 patients with breast cancer before, during, and after adjuvant therapy for the non-metastatic disease, or during therapy for metastatic disease. Patients showed a marked reduction in peak oxygen consumption (VO2), especially those with metastatic disease, but also those before therapy, compared to patients after adjuvant therapy and age-matched sedentary healthy women. Peak VO2 was an independent predictor of survival, with an adjusted hazard ratio (HR) for death of 0.59 for a VO2 peak ≥ 15.4 mL/kg/min (95% CI, 0.29–1.19; p = 0.14) [111].
Overall, there is limited evidence of cardiac functional impairment of untreated cancer patients, and findings are basically limited to breast cancer patients.
Autonomic function
Resting heart rate has a strong association with cancer mortality [112,113,114]. In a study on 548 treatment-naïve cancer patients, higher resting heart rate was associated with higher NT-proBNP and hs-TnT, and predicted all-cause mortality over a median of 25 months after adjustment for age, gender, tumour type and stage, cardiac status, and haemoglobin (HR for each 5 beats per minute increase 1.10; 95% CI, 1.04–1.16; p < 0.001). The strongest associations with mortality were observed in lung and gastrointestinal cancer (p = 0.007 and p < 0.001, respectively) [115].
Assessment of heart rate variability (HRV), evaluated through ECG Holter monitoring, allows a more comprehensive evaluation of autonomic function. Reduced HRV denotes autonomic dysfunction with sympathetic activation and vagal withdrawal and portends a worse prognosis in several conditions, from cardiovascular to neurodegenerative diseases [116,117,118]. HRV is reduced in several cancer types [119], and decreased with advanced clinical stage and tumour progression (both p < 0.001) in patients with gastric cancer [120]. Cramer et al. prospectively studied 50 patients with CRC, 51 patients with HF, and 51 control subjects. Most metrics of HRV were significantly reduced in CRC patients and HF patients compared with control subjects (all p < 0.05).
In summary, cancer patients have a higher heart rate and a reduced HRV, with clinical and prognostic implications.
Therapeutic perspectives
Several therapies with antifibrotic and antiremodelling effect (mainly RAAS antagonists), autonomic modulation (beta-blockers), and antiinflammatory or antioxidant actions [78, 80] have shown to prevent or relieve chemotherapy cardiotoxicity [121]. Exercise training seems also protective [110, 122, 123], for example reducing NT-proBNP increase and systolic dysfunction in breast cancer patient receiving doxorubicin [124] (Table 2).
Beta-blockers
Patients on beta-blockers before cancer diagnosis tend to show a reduced disease progression and mortality [125,126,127,128]. Indeed, beta-blockers limit (mainly via beta-2-adrenergic receptors) inflammation and metastasis formation [59, 129]. Beta-blockers may then represent an adjuvant therapy strategy with a pleiotropic impact on the primary tumour, its microenvironment, and metastasis formation [128,129,130].
Powe et al. hypothesised a better outcome for breast cancer patients receiving a beta-blocker for hypertension. They evaluated 3 patient subgroups of 466 consecutive female patients (43 treated with beta-blockers, 49 with other antihypertensives, and 374 non-hypertensive control subgroup) with resectable breast cancer and follow-up of > 10 years. The endpoints were breast cancer survival, disease-free survival, formation of distant metastases, and local tumour recurrence. Patients on beta-blockers showed a significant reduction in metastasis development (p = 0.026), tumour recurrence (p = 0.001), and longer disease-free survival (p = 0.01). Furthermore, the risk of metastasis formation and breast cancer mortality at 10 years were reduced by 57% and 71%, respectively [125].
A recent meta-analysis of 12 studies and more than 20,000 patients reported that beta-blocker therapy is associated with improved overall survival (HR 0.79; 95% CI, 0.67–0.93; p = 0.004) and disease-free survival (HR 0.69; 95% CI, 0.53–0.91; p = 0.009). The effect size was greater, albeit not significantly different, in patients with low-stage cancer or cancer treated primarily with surgery [131].
RAAS inhibitors
Angiotensin-converting enzyme inhibitor (ACEi) therapy was originally found to be independently associated with a decreased risk for cancer occurrence in a population-based study including hypertensive patients (HR 0.66; 95% CI, 0.63–0.68; p < 0.001) [132]. Studies on larger populations reported a lower cumulative incidence of cancer for angiotensin receptor blocker (ARB) users (HR 0.58, 95% CI 0.55–0.62; p 0.001) [133, 134]. RAAS blockade has also been associated with better survival in patients with metastatic renal cell carcinoma [135]. Despite this, several meta-analyses have yielded conflicting results regarding the association between ARB therapy and the risk of new diagnosis of cancer [136,137,138].
A few studies investigated the effect of beta blockers and RAAS inhibitors. In the LACE Study cohort [women with early-stage breast cancer from the Kaiser Permanente Northern California Cancer Registry], including 1779 women (with 292 cancer recurrences, 174 cancer deaths, and 323 total deaths), 23% of patients were treated with either a beta-blocker and/or ACEi. These drugs were associated with older age, postmenopausal status, tamoxifen therapy, higher body mass index, hypertension, and diabetes. ACEi therapy was surprisingly associated with breast cancer recurrence (HR 1.56; 95% CI, 1.02–2.39; p = 0.04), but not cause-specific mortality or overall mortality. On the contrary, beta-blocker therapy was associated with a lower hazard of recurrence and cause-specific mortality (HR 0.86; 95% CI, 0.57–1.32; p = 0.49). However, there was no evidence of dose response with either medication. For recurrence and cause-specific mortality, therapy with a beta-blocker and an ACEi was associated with a lower HR for the outcome (HR 1.14 and 1.04, for the respective outcome) than when ACEi alone was used (HR 1.56 and 1.27, respectively) [139].
A further retrospective study including a large population of 18,733 women diagnosed with non-metastatic breast cancer between 1996 and 2003 showed that users of any beta-blocker had a lower recurrence hazard in unadjusted models (HR 0.91; 95% CI, 0.81–1.0) and a slightly higher recurrence hazard in adjusted models (adjusted HR 1.3; 95% CI, 1.1–1.5), with similar associations for exposures defined by receptor selectivity and solubility. Metoprolol and sotalol were associated with increased recurrence rates (adjusted HR: 1.5 metoprolol, 2.0 sotalol). ACEi were associated with a slightly increased recurrence hazard, whereas angiotensin II receptor blockers (ARBs) were not associated with recurrence. The authors concluded that the study did not support the hypothesis that beta-blockers reduced the risk of breast cancer recurrence [140].
Retrospective cohort studies might have influenced these results, as there are no randomized controlled trials yet investigating this subject. Also, the studies used various beta blockers with different types of subjects in different tumour settings, leading to significant heterogeneity in the results.
Other drugs
Some other cardioactive drugs are currently under investigation: while treatment with diuretics does not seem to affect tumour incidence [141], the use of statins [142], aspirin [143], and metformin [144] seems to lead to a lower risk of death and tumour incidence.
Ongoing studies
Several ongoing studies are investigating the potential implementation of beta-blockers in this specific setting (notably propranolol and carvedilol) [7].
Although various studies have suggested that ACEi/ARB have antiproliferative effects and improve survival of many types of cancers, there are also reports of increased cancer risk: because of contradictory findings from meta-analyses, the exact relationship between RAAS blockade and development of cancer and cancer subtypes remains uncertain and more studies (longitudinal prospective cohort; randomized clinical trials) are needed to demonstrate the effects of RAAS blockade in cancer, and in this setting, large randomized controlled trials are mandatory.
Conclusions
Cancer itself might be considered as a condition at risk of HF and, as such, susceptible to cardioprotective therapies even before the introduction of chemotherapy. Such primary prevention strategies would allow to avoid further complications and better risk stratify cancer patients, but, on the other hand, they imply greater risk of side effects, closer monitoring, and higher cost (Fig. 2). Overall, there are promising results from primary prevention trials investigating the cardioprotective efficacy of neurohormonal therapies [145,146,147,148,149,150,151]. Breast cancer patients treated with neurohormonal therapies show higher LVEF and better LV strain [121]. However, these trials have highly heterogeneous designs. The small sample size, short follow-up durations, and single-centred design highlight the need for multicentre, adequately powered randomized controlled trials. Longer follow-up duration and clinically meaningful endpoints are also required [121, 152].
Therefore, patients candidate for chemotherapy should be considered at risk of developing HF (stage A), and subclinical HF (stage B) that should be actively searched, to Initiate early an appropriate treatment and improve patient outcome.
References
Siegel RL, Miller KD, Fuchs H, Jemal A (2021) Cancer Statistics, 2021. CA Cancer J Clin 71:7–33. https://doi.org/10.3322/caac.21654
Cardinale D, Biasillo G, Cipolla CM (2016) Curing cancer saving the heart: a challenge that cardioncology should not miss. Curr Cardiol Rep 18(6):51
de Boer RA, Meijers WC, van der Meer P, van Veldhuisen DJ (2019) Cancer and heart disease: associations and relations. Eur J Heart Fail 21(12):1515–1525
Meijers WC, de Boer RA (2019) Common risk factors for heart failure and cancer. Cardiovasc Res 115(5):844–853
Koene RJ, Prizment AE, Blaes A, Konety SH (2016) Shared risk factors in cardiovascular disease and cancer. Circulation 133(11):1104–1114
Tu H, Wen CP, Tsai SP, Chow WH, Wen C, Ye Y et al (2018) Cancer risk associated with chronic diseases and disease markers: prospective cohort study. BMJ 360:134
de Boer RA, Hulot JS, Gabriele Tocchetti C, Aboumsallem JP, Ameri P, Anker SD et al (2020) Common mechanistic pathways in cancer and heart failure. Eur J Heart Fail 22(12):2272–2289
Karlstaedt A, Zhang X, Vitrac H, Harmancey R, Vasquez H, Wang JH et al (2016) Oncometabolite d-2-hydroxyglutarate impairs alpha-ketoglutarate dehydrogenase and contractile function in rodent heart. Proc Natl Acad Sci U S A 113(37):10436–10441
Cuomo A, Pirozzi F, Attanasio U, Franco R, Elia F, De Rosa E et al (2020) Cancer risk in the heart failure population: epidemiology mechanisms and clinical implications. Curr Oncol Rep 23(1):7
Strongman H, Gadd S, Matthews A, Mansfield KE, Stanway S, Lyon AR et al (2019) Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet 394(10203):1041–1054
Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, Gammon MD (2016) Cardiovascular disease mortality among breast cancer survivors. Epidemiology 27(1):6–13
Chow EJ, Chen Y, Hudson MM, Feijen EAM, Kremer LC, Border WL et al (2018) Prediction of ischemic heart disease and stroke in survivors of childhood cancer. J Clin Oncol 36(1):44–52
Cuddy SAM, Falk RH (2020) Amyloidosis as a systemic disease in context. Can J Cardiol 36(3):396–407
Zhang R, Gupta D, Albert SG (2017) Pheochromocytoma as a reversible cause of cardiomyopathy: analysis and review of the literature. Int J Cardiol 249:319–323
Mobine HR, Baker AB, Wang L, Wakimoto H, Jacobsen KC, Seidman CE et al (2009) Pheochromocytoma-induced cardiomyopathy is modulated by the synergistic effects of cell-secreted factors. Circ Heart Fail 2(2):121–128
Dero I, De Pauw M, Borbath I, Delaunoit T, Demetter P, Demolin G et al (2009) Carcinoid heart disease—a hidden complication of neuroendocrine tumours. Acta gastro-enterol Belg 72(1):34–38
Vitale G, Pivonello R, Lombardi G, Colao A (2004) Cardiac abnormalities in acromegaly Treat Endocrinol 3(5):309–318
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454(7203):436–444
Zhang S, Yang X, Wang L, Zhang C (2018) Interplay between inflammatory tumor microenvironment and cancer stem cells. Oncol Lett 16(1):679–686
Gunter MJ, Stolzenberg-Solomon R, Cross AJ, Leitzmann MF, Weinstein S, Wood RJ et al (2006) A prospective study of serum C-reactive protein and colorectal cancer risk in men. Cancer Res 66(4):2483–2487
Xu J, Ye Y, Zhang H, Szmitkowski M, Makinen MJ, Li P et al (2016) Diagnostic and prognostic value of serum interleukin-6 in colorectal cancer. Medicine 95(2):e2502
Yan G, Liu T, Yin L, Kang Z, Wang L (2018) Levels of peripheral Th17 cells and serum Th17-related cytokines in patients with colorectal cancer: a meta-analysis. Cell Mol Biol 64(6):94–102
Müerköster S, Wegehenkel K, Arlt A, Witt M, Sipos B, Kruse ML et al (2004) Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interleukin-1beta. Cancer Res 64(4):1331–1337
Bertero E, Canepa M, Maack C, Ameri P (2018) Linking heart failure to cancer: background evidence and research perspectives. Circulation 138(7):735–742
Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ et al (2017) Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised double-blind placebo-controlled trial. Lancet 390(10105):1833–1842
Canli O, Nicolas AM, Gupta J, Finkelmeier F, Goncharova O, Pesic M et al (2017) Myeloid cell-derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell 32(6):869–883
Hayes JD, Dinkova-Kostova AT, Tew KD (2020) Oxidative stress in cancer. Cancer Cell 38(2):167–197
Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD (2019) ROS and the DNA damage response in cancer. Redox Biol 25:101084
Waris G, Ahsan H (2006) Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog 5:14
Maya-Mendoza A, Ostrakova J, Kosar M, Hall A, Duskova P, Mistrik M et al (2015) Myc and Ras oncogenes engage different energy metabolism programs and evoke distinct patterns of oxidative and DNA replication stress. Mol Oncol 9(3):601–616
Aimo A, Castiglione V, Borrelli C, Saccaro LF, Franzini M, Masi S et al (2020) Oxidative stress and inflammation in the evolution of heart failure: from pathophysiology to therapeutic strategies. Eur J Prev Cardiol 27(5):494–510
Tsutsui H, Kinugawa S, Matsushima S (2011) Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 301(6):H2181–H2190
Ager EI, Neo J, Christophi C (2008) The renin-angiotensin system and malignancy. Carcinogenesis 29(9):1675–1684
Hanif K, Bid HK, Konwar R (2010) Reinventing the ACE inhibitors: some old and new implications of ACE inhibition. Hypertens Res 33(1):11–21
Deshayes F, Nahmias C (2005) Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab 16(7):293–299
Daemen MJ, Lombardi DM, Bosman FT, Schwartz SM (1991) Angiotensin II induces smooth muscle cell proliferation in the normal and injured rat arterial wall. Circ Res 68(2):450–456
Buharalioglu CK, Song CY, Yaghini FA, Ghafoor HU, Motiwala M, Adris T et al (2011) Angiotensin II-induced process of angiogenesis is mediated by spleen tyrosine kinase via VEGF receptor-1 phosphorylation. Am J Physiol Heart Circ Physiol 301(3):H1043–H1055
Li SH, Lu HI, Chang AY, Huang WT, Lin WC, Lee CC et al (2016) Angiotensin II type I receptor (AT1R) is an independent prognosticator of esophageal squamous cell carcinoma and promotes cells proliferation via mTOR activation. Oncotarget 7(41):67150–67165
Afify SM, Seno M (2019) Conversion of stem cells to cancer stem cells: undercurrent of cancer initiation. Cancer 11(3):345
Munro MJ, Wickremesekera AC, Davis PF, Marsh R, Tan ST (2017) Renin-angiotensin system and cancer: a review. Integr Cancer Sci Therap 4(2):1–6
Unger T, Li J (2004) The role of the renin-angiotensin-aldosterone system in heart failure. J Renin Angiotensin Aldosterone Syst 5(Suppl 1):S7-10
Sayer G, Bhat G (2014) The renin-angiotensin-aldosterone system and heart failure. Cardiol Clin 32(1):21–32
Argyriou AA, Bruna J, Marmiroli P, Cavaletti G (2012) Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol 82(1):51–77
Coumbe BGT, Groarke JD (2018) Cardiovascular autonomic dysfunction in patients with cancer. Curr Cardiol Rep 20(8):69
Teng AE, Noor B, Ajijola OA, Yang EH (2021) Chemotherapy and radiation-associated cardiac autonomic dysfunction. Curr Oncol Rep 23(2):14
Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ et al (2013) Autonomic nerve development contributes to prostate cancer progression. Science 341(6142):1236361
Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W et al (2011) A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature 477(7364):349–353
Nilsson MB, Armaiz-Pena G, Takahashi R, Lin YG, Trevino J, Li Y et al (2007) Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. J Biol Chem 282(41):29919–29926
Shahzad MM, Arevalo JM, Armaiz-Pena GN, Lu C, Stone RL, Moreno-Smith M et al (2010) Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J Biol Chem 285(46):35462–35470
Bucsek MJ, Qiao G, MacDonald CR, Giridharan T, Evans L, Niedzwecki B et al (2017) β-adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8(+) T cells and undermines checkpoint inhibitor therapy. Cancer Res 77(20):5639–5651
Nissen MD, Sloan EK, Mattarollo SR (2018) Beta-adrenergic signaling impairs antitumor CD8(+) T-cell responses to B-cell lymphoma immunotherapy. Cancer Immunol Res 6(1):98–109
Sood AK, Armaiz-Pena GN, Halder J, Nick AM, Stone RL, Hu W et al (2010) Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest 120(5):1515–1523
Li Y, Yang S, Sadaoui NC, Hu W, Dasari SK, Mangala LS et al (2020) Sustained adrenergic activation of YAP1 induces anoikis resistance in cervical cancer cells. iScience 23(7):101289
Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C et al (2006) Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 12(8):939–944
Yang EV, Sood AK, Chen M, Li Y, Eubank TD, Marsh CB et al (2006) Norepinephrine up-regulates the expression of vascular endothelial growth factor matrix metalloproteinase (MMP)-2 and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res 66(21):10357–10364
Cole SW, Sood AK (2012) Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res 18(5):1201–1206
Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V et al (2010) The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 70(18):7042–7052
Partecke LI, Speerforck S, Kading A, Seubert F, Kuhn S, Lorenz E et al (2016) Chronic stress increases experimental pancreatic cancer growth reduces survival and can be antagonised by beta-adrenergic receptor blockade. Pancreatology 16(3):423–433
Nagaraja AS, Sadaoui NC, Lutgendorf SK, Ramondetta LM, Sood AK (2013) β-blockers: a new role in cancer chemotherapy? Expert Opin Investig Drugs 22(11):1359–1363
Peixoto R, Pereira MdL, Oliveira M (2020) Beta-blockers and cancer: where are we? Pharmaceuticals 13(6):105
Floras JS, Ponikowski P (2015) The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur Heart J 36(30):1974–1982
Vergaro G, Aimo A, Prontera C, Ghionzoli N, Arzilli C, Zyw L et al (2019) Sympathetic and renin-angiotensin-aldosterone system activation in heart failure with preserved mid-range and reduced ejection fraction. Int J Cardiol 296:91–97
van Bilsen M, Patel HC, Bauersachs J, Böhm M, Borggrefe M, Brutsaert D et al (2017) The autonomic nervous system as a therapeutic target in heart failure: a scientific position statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 19(11):1361–1378
Gibson CJ, Steensma DP (2018) New insights from studies of clonal hematopoiesis. Clin Cancer Res 24(19):4633–4642
Acuna-Hidalgo R, Sengul H, Steehouwer M, van de Vorst M, Vermeulen SH, Kiemeney L et al (2017) Ultra-sensitive sequencing identifies high prevalence of clonal hematopoiesis-associated mutations throughout adult life. Am J Hum Genet 101(1):50–64
Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E et al (2017) Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. New Engl J Med 377(2):111–121
Libby P (2017) Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J Am Coll Cardiol 70(18):2278–2289
Dorsheimer L, Assmus B, Rasper T, Ortmann CA, Ecke A, Abou-El-Ardat K et al (2019) Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol 4(1):25–33
Cremer S, Schloss MJ, Vinegoni C, Foy BH, Zhang S, Rohde D et al (2020) Diminished reactive hematopoiesis and cardiac inflammation in a mouse model of recurrent myocardial infarction. J Am Coll Cardiol 75(8):901–915
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG et al (2014) Age-related clonal hematopoiesis associated with adverse outcomes. New Engl J Med 371(26):2488–2498
Collins RRJ, Patel K, Putnam WC, Kapur P, Rakheja D (2017) Oncometabolites: a new paradigm for oncology metabolism and the clinical laboratory. Clin Chem 63(12):1812–1820
Akbay EA, Moslehi J, Christensen CL, Saha S, Tchaicha JH, Ramkissoon SH et al (2014) D-2-hydroxyglutarate produced by mutant IDH2 causes cardiomyopathy and neurodegeneration in mice. Genes Dev 28(5):479–490
Liberti MV, Locasale JW (2016) The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 41(3):211–218
Chen Z, Liu M, Li L, Chen L (2018) Involvement of the Warburg effect in non-tumor diseases processes. J Cell Physiol 233(4):2839–2849
Taegtmeyer H, Karlstaedt A, Rees ML, Davogustto G (2017) Oncometabolic tracks in the heart. Circ Res 120(2):267–269
Rees ML, Subramaniam J, Li Y, Hamilton DJ, Frazier OH, Taegtmeyer H (2015) A PKM2 signature in the failing heart. Biochem Biophys Res Commun 459(3):430–436
Sarhene M, Wang Y, Wei J, Huang Y, Li M, Li L et al (2019) Biomarkers in heart failure: the past, current and future. Heart Fail Rev 24(6):867–903
Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A et al (2020) Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol 31(2):171–190
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M et al (2014) Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 15(10):1063–1093
Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M et al (2016) 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 37(36):2768–2801
Lyon AR (2015) Disparate worlds drawing closer together: cardiovascular biomarkers predict cancer outcomes in treatment-naive patients. Heart 101(23):1853–1854
Nikitenko LL, Fox SB, Kehoe S, Rees MC, Bicknell R (2006) Adrenomedullin and tumour angiogenesis. Br J Cancer 94(1):1–7
Nelson J, Bagnato A, Battistini B, Nisen P (2003) The endothelin axis: emerging role in cancer. Nat Rev Cancer 3(2):110–116
Law C, Glover C, Benson K, Guglin M (2010) Extremely high brain natriuretic peptide does not reflect the severity of heart failure. Congest Heart Fail 16(5):221–225
Wigle DA, Campling BG, Sarda IR, Shin SH, Watson JD, Frater Y et al (1995) ANP secretion from small cell lung cancer cell lines: a potential model of ANP release. Am J Physiol 268(5):H1869–H1874
Ohsaki Y, Gross AJ, Le PT, Oie H, Johnson BE (1999) Human small cell lung cancer cells produce brain natriuretic peptide. Oncology 56(2):155–159
Popat J, Rivero A, Pratap P, Guglin M (2013) What is causing extremely elevated amino terminal brain natriuretic peptide in cancer patients? Congest Heart Fail 19(3):143–148
Petricciuolo S, Delle Donne MG, Aimo A, Chella A, De Caterina R (2021) Pre-treatment high-sensitivity troponin T for the short-term prediction of cardiac outcomes in patients on immune checkpoint inhibitors. Eur J Clin Invest 51(4):e13400
Florido R, Lee AK, McEvoy JW, Hoogeveen RC, Koton S, Vitolins MZ et al (2019) Cancer survivorship and subclinical myocardial damage. Am J Epidemiol 188(12):2188–2195
Missov E, Calzolari C, Davy JM, Leclercq F, Rossi M, Pau B (1997) Cardiac troponin I in patients with hematologic malignancies. Coron Artery Dis 8(8–9):537–541
Cramer L, Hildebrandt B, Kung T, Wichmann K, Springer J, Doehner W et al (2014) Cardiovascular function and predictors of exercise capacity in patients with colorectal cancer. J Am Coll Cardiol 64(13):1310–1319
Pavo N, Raderer M, Hulsmann M, Neuhold S, Adlbrecht C, Strunk G et al (2015) Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart 101(23):1874–1880
Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D et al (2016) Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin 66(4):309–325
Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M et al (2017) 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail 19(1):9–42
Mornos C, Manolis AJ, Cozma D, Kouremenos N, Zacharopoulou I, Ionac A (2014) The value of left ventricular global longitudinal strain assessed by three-dimensional strain imaging in the early detection of anthracyclinemediated cardiotoxicity. Hellenic J Cardiol 55(3):235–244
Narayan HK, Finkelman B, French B, Plappert T, Hyman D, Smith AM et al (2017) Detailed echocardiographic phenotyping in breast cancer patients: associations with ejection fraction decline, recovery, and heart failure symptoms over 3 years of follow-up. Circulation 135(15):1397–1412
Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH (2014) Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 63(25 Pt A):2751–2768
Brown J, Jenkins C, Marwick TH (2009) Use of myocardial strain to assess global left ventricular function: a comparison with cardiac magnetic resonance and 3-dimensional echocardiography. Am Heart J 157(1):102-e1
Liu J, Banchs J, Mousavi N, Plana JC, Scherrer-Crosbie M, Thavendiranathan P et al (2018) Contemporary role of echocardiography for clinical decision making in patients during and after cancer therapy. JACC Cardiovasc Imaging 11(8):1122–31
Tadic M, Genger M, Baudisch A, Kelle S, Cuspidi C, Belyavskiy E et al (2018) Left ventricular strain in chemotherapy-naive and radiotherapy-naive patients with cancer. Can J Cardiol 34(3):281–7
Tadic M, Baudisch A, Haßfeld S, Heinzel F, Cuspidi C, Burkhardt F et al (2018) Right ventricular function and mechanics in chemotherapy- and radiotherapy-naïve cancer patients. Int J Cardiovasc Imaging 34(10):1581–7
Jeong D, Gladish G, Chitiboi T, Fradley MG, Gage KL, Schiebler ML (2019) MRI in cardio-oncology: a review of cardiac complications in oncologic care. J Magn Reson Imaging 50(5):1349–66
Thavendiranathan P, Wintersperger BJ, Flamm SD, Marwick TH (2013) Cardiac MRI in the assessment of cardiac injury and toxicity from cancer chemotherapy: a systematic review. Circ Cardiovasc Imaging 6(6):1080–91
Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Choudhury RP, et al (2012) Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson 14:42
Galán-Arriola C, Lobo M, Vílchez-Tschischke JP, López GJ, de Molina-Iracheta A, Pérez-Martínez C et al (2019) Serial magnetic resonance imaging to identify early stages of anthracycline-induced cardiotoxicity. J Am Coll Cardiol 73(7):779–91
Meléndez GC, Jordan JH, D’Agostino RB Jr, Vasu S, Hamilton CA, Hundley WG (2017) Progressive 3-month increase in LV myocardial ECV after anthracycline-based chemotherapy. JACC Cardiovasc Imaging 10(6):708–9
Haslbauer JD, Lindner S, Valbuena-Lopez S, Zainal H, Zhou H, D’Angelo T et al (2019) CMR imaging biosignature of cardiac involvement due to cancer-related treatment by T1 and T2 mapping. Int J Cardiol 275:179–86
Jones LW, Liang Y, Pituskin EN, Battaglini CL, Scott JM, Hornsby WE et al (2011) Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist 16(1):112–20
Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM et al (2009) Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol 27(27):4605–12
Howden EJ, Bigaran A, Beaudry R, Fraser S, Selig S, Foulkes S et al (2019) Exercise as a diagnostic and therapeutic tool for the prevention of cardiovascular dysfunction in breast cancer patients. Eur J Prev Cardiol 26(3):305–15
Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM et al (2012) Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol 30(20):2530–7
Persky V, Dyer AR, Leonas J, Stamler J, Berkson DM, Lindberg HA et al (1981) Heart rate: a risk factor for cancer? Am J Epidemiol 114(4):477–87
Thomas F, Guize L, Bean K, Benetos A (2001) Pulse pressure and heart rate: independent risk factors for cancer? J Clin Epidemiol 54(7):735–40
Jouven X, Escolano S, Celermajer D, Empana JP, Bingham A, Hermine O et al (2011) Heart rate and risk of cancer death in healthy men. PLoS One 6(8):e21310
Anker MS, Frey MK, Goliasch G, Bartko PE, Prausmuller S, Gisslinger H et al (2020) Increased resting heart rate and prognosis in treatment-naive unselected cancer patients: results from a prospective observational study. Eur J Heart Fail 22(7):1230–1238
Shaffer F, Ginsberg JP (2017) An overview of heart rate variability metrics and norms. Front Public Health 5:258
Catai AM, Pastre CM, Godoy MF, Silva ED, Takahashi ACM, Vanderlei LCM (2020) Heart rate variability: are you using it properly Standardisation checklist of procedures. Braz J Phys Ther 24(2):91–102
Vanderlei LC, Pastre CM, Hoshi RA, Carvalho TD, Godoy MF (2009) Basic notions of heart rate variability and its clinical applicability. Braz J Cardiovasc Surg 24(2):205–17
Kloter E, Barrueto K, Klein SD, Scholkmann F, Wolf U (2018) Heart rate variability as a prognostic factor for cancer survival—a systematic review. Front Physiol 9:623
Hu S, Lou J, Zhang Y, Chen P (2018) Low heart rate variability relates to the progression of gastric cancer. World J Surg Oncol 16(1):49
Vaduganathan M, Hirji SA, Qamar A, Bajaj N, Gupta A, Zaha VG, Chandra A, Haykowsky M, Ky B, Moslehi J, Nohria A, Butler J, Pandey A (2019) Efficacy of neurohormonal therapies in preventing cardiotoxicity in patients with cancer undergoing chemotherapy. J Am Coll Cardiol CardioOnc 1(1):54–65
Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM et al (2010) American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 42(7):1409–26
Scott JM, Nilsen TS, Gupta D, Jones LW (2018) Exercise therapy and cardiovascular toxicity in cancer. Circulation 137(11):1176–91
Kirkham AA, Shave RE, Bland KA, Bovard JM, Eves ND, Gelmon KA et al (2017) Protective effects of acute exercise prior to doxorubicin on cardiac function of breast cancer patients: a proof-of-concept RCT. Int J Cardiol 245:263–70
Powe DG, Voss MJ, Zänker KS, Habashy HO, Green AR, Ellis IO et al (2010) Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 1(7):628–38
De Giorgi V, Grazzini M, Gandini S, Benemei S, Lotti T, Marchionni N et al (2011) Treatment with β-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med 171(8):779–81
Grytli HH, Fagerland MW, Fosså SD, Taskén KA, Håheim LL (2013) Use of β-blockers is associated with prostate cancer-specific survival in prostate cancer patients on androgen deprivation therapy. Prostate 73(3):250–60
Montoya A, Varela-Ramirez A, Dickerson E, Pasquier E, Torabi A, Aguilera R et al (2019) The beta adrenergic receptor antagonist propranolol alters mitogenic and apoptotic signaling in late stage breast cancer. Biomed J 42(3):155–65
Ji Y, Chen S, Xiao X, Zheng S, Li K (2012) β-blockers: a novel class of antitumor agents. Onco Targets Ther 5:391–401
Bustamante P, Miyamoto D, Goyeneche A, de Alba Graue PG, Jin E, Tsering T et al (2019) Beta-blockers exert potent anti-tumor effects in cutaneous and uveal melanoma. Cancer Med 8(17):7265–77
Choi CH, Song T, Kim TH, Choi JK, Park JY, Yoon A et al (2014) Meta-analysis of the effects of beta blocker on survival time in cancer patients. J Cancer Res Clin Oncol 140(7):1179–88
Huang CC, Chan WL, Chen YC, Chen TJ, Lin SJ, Chen JW et al (2011) Angiotensin II receptor blockers and risk of cancer in patients with systemic hypertension. Am J Cardiol 107(7):1028–33
Rao GA, Mann JR, Shoaibi A, Pai SG, Bottai M, Sutton SS et al (2013) Angiotensin receptor blockers: are they related to lung cancer? J Hypertens 31(8):1669–75
Wang KL, Liu CJ, Chao TF, Huang CM, Wu CH, Chen TJ et al (2013) Long-term use of angiotensin II receptor blockers and risk of cancer: a population-based cohort analysis. Int J Cardiol 167(5):2162–6
McKay RR, Rodriguez GE, Lin X, Kaymakcalan MD, Hamnvik OP, Sabbisetti VS et al (2015) Angiotensin system inhibitors and survival outcomes in patients with metastatic renal cell carcinoma. Clin Cancer Res 21(11):2471–9
Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC (2010) Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol 11(7):627–36
Bangalore S, Kumar S, Kjeldsen SE, Makani H, Grossman E, Wetterslev J et al (2011) Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324168 participants from randomised trials. Lancet Oncol 12(1):65–82
Sipahi I, Chou J, Mishra P, Debanne SM, Simon DI, Fang JC (2011) Meta-analysis of randomized controlled trials on effect of angiotensin-converting enzyme inhibitors on cancer risk. Am J Cardiol 108(2):294–301
Ganz PA, Habel LA, Weltzien EK, Caan BJ, Cole SW (2011) Examining the influence of beta blockers and ACE inhibitors on the risk for breast cancer recurrence: results from the LACE cohort. Breast Cancer Res Treat 129(2):549–56
Sorensen GV, Ganz PA, Cole SW, Pedersen LA, Sorensen HT, Cronin-Fenton DP et al (2013) Use of beta-blockers angiotensin-converting enzyme inhibitors angiotensin II receptor blockers and risk of breast cancer recurrence: a Danish nationwide prospective cohort study. J Clin Oncol 31(18):2265–72
Coogan PF, Strom BL, Rosenberg L (2009) Diuretic use and the risk of breast cancer. J Hum Hypertens 23(3):216–8
Di Bello E, Zwergel C, Mai A, Valente S (2020) The innovative potential of statins in cancer: new targets for new therapies. Front Chem 8:516
Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW (2011) Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377(9759):31–41
Soranna D, Scotti L, Zambon A, Bosetti C, Grassi G, Catapano A et al (2012) Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist 17(6):813–22
Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M et al (2006) Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 114(23):2474–81
Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW et al (2016) Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial randomized placebo-controlled double-blind clinical trial of candesartan and metoprolol. Eur Heart J 37(21):1671–80
Pituskin E, Mackey JR, Koshman S, Jassal D, Pitz M, Haykowsky MJ et al (2017) Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101-Breast): a randomized trial for the prevention of trastuzumab-associated cardiotoxicity. J Clin Oncol 35(8):870–7
Guglin M, Krischer J, Tamura R, Fink A, Bello-Matricaria L, McCaskill-Stevens W et al (2019) Randomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J Am Coll Cardiol 73(22):2859–68
Lee M, Chung WB, Lee JE, Park CS, Park WC, Song BJ et al (2021) Candesartan and carvedilol for primary prevention of subclinical cardiotoxicity in breast cancer patients without a cardiovascular risk treated with doxorubicin. Cancer Med 10(12):3964–3973
Avila MS, Ayub-Ferreira SM, de Barros Wanderley Jr MR, das Dores Cruz F, Goncalves Brandao SM, Rigaud VOC, et al (2018) Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol 71(20):2281–90
Boekhout AH, Gietema JA, Milojkovic Kerklaan B, van Werkhoven ED, Altena R, Honkoop A et al (2016) Angiotensin II-receptor inhibition with candesartan to prevent trastuzumab-related cardiotoxic effects in patients with early breast cancer: a randomized clinical trial. JAMA Oncol 2(8):1030–7
Kikuchi R, Shah NP, Dent SF (2020) Strategies to prevent cardiovascular toxicity in breast cancer: is it ready for primetime? J Clin Med 9(4):896
Funding
Open access funding provided by Scuola Superiore Sant'Anna within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
I.F., G.P., and A.G.: searched the literature and drafted tables and figures. I.F., G.P., A.A., A.G., and D.M.C.: drafted the paper. C.G., G.V., N.R.P., C.P., S.T., and M.E.: edited and revised critically the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Iacopo Fabiani and Giorgia Panichella equally contributed and are joint first authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fabiani, I., Panichella, G., Aimo, A. et al. Subclinical cardiac damage in cancer patients before chemotherapy. Heart Fail Rev 27, 1091–1104 (2022). https://doi.org/10.1007/s10741-021-10151-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-021-10151-4