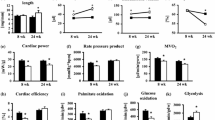
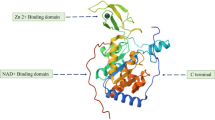
Abstract
Sirtuins (SIRTs) are NAD+-dependent enzymes that catalyze deacylation of protein lysine residues. In mammals, seven sirtuins have been identified, SIRT1–7. SIRT3–5 are mainly or exclusively localized within mitochondria and mainly participate in the regulation of energy metabolic pathways. Since mitochondrial ATP regeneration is inevitably linked to the maintenance of cardiac pump function, it is not surprising that recent studies revealed a role for mitochondrial sirtuins in the regulation of myocardial energetics and function. In addition, mitochondrial sirtuins modulate the extent of myocardial ischemia reperfusion injury and the development of cardiac hypertrophy and failure. Thus, targeting mitochondrial sirtuins has been proposed as a novel approach to improve myocardial mitochondrial energetics, which is frequently impaired in cardiac disease and considered an important underlying cause contributing to several cardiac pathologies, including myocardial ischemia reperfusion injury and heart failure. In the current review, we present and discuss the available literature on mitochondrial sirtuins and their potential roles in cardiac physiology and disease.

Similar content being viewed by others
References
Stanley WC, Recchia FA, Lopaschuk GD (2005) Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85(3):1093–1129. doi:10.1152/physrev.00006.2004
Keating ST, El-Osta A (2015) Epigenetics and metabolism. Circ Res 116(4):715–736. doi:10.1161/CIRCRESAHA.116.303936
Guarente L, Picard F (2005) Calorie restriction—the SIR2 connection. Cell 120(4):473–482. doi:10.1016/j.cell.2005.01.029
Imai S, Armstrong CM, Kaeberlein M, Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403(6771):795–800. doi:10.1038/35001622
Rogina B, Helfand SL (2004) Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA 101(45):15998–16003. doi:10.1073/pnas.0404184101
Kaeberlein M, McVey M, Guarente L (1999) The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev 13(19):2570–2580
Tissenbaum HA, Guarente L (2001) Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410(6825):227–230. doi:10.1038/3506563835065638
Mathias RA, Greco TM, Oberstein A, Budayeva HG, Chakrabarti R, Rowland EA, Kang Y, Shenk T, Cristea IM (2014) Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell 159(7):1615–1625. doi:10.1016/j.cell.2014.11.046
Tan M, Peng C, Anderson KA, Chhoy P, Xie Z, Dai L, Park J, Chen Y, Huang H, Zhang Y, Ro J, Wagner GR, Green MF, Madsen AS, Schmiesing J, Peterson BS, Xu G, Ilkayeva OR, Muehlbauer MJ, Braulke T, Muhlhausen C, Backos DS, Olsen CA, McGuire PJ, Pletcher SD, Lombard DB, Hirschey MD, Zhao Y (2014) Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab 19(4):605–617. doi:10.1016/j.cmet.2014.03.014
Hirschey MD, Zhao Y (2015) Metabolic regulation by lysine malonylation, succinylation, and glutarylation. Mol Cell Proteom MCP 14(9):2308–2315. doi:10.1074/mcp.R114.046664
Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B, Chen W, Zhang S, Cerione RA, Auwerx J, Hao Q, Lin H (2011) Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334(6057):806–809. doi:10.1126/science.1207861
Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y (2006) Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell 23(4):607–618. doi:10.1016/j.molcel.2006.06.026
Park J, Chen Y, Tishkoff DX, Peng C, Tan M, Dai L, Xie Z, Zhang Y, Zwaans BM, Skinner ME, Lombard DB, Zhao Y (2013) SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell 50(6):919–930. doi:10.1016/j.molcel.2013.06.001
Bugger H, Abel ED (2014) Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 57(4):660–671. doi:10.1007/s00125-014-3171-6
Murphy E, Steenbergen C (2008) Mechanisms underlying acute protection from cardiac ischemia–reperfusion injury. Physiol Rev 88(2):581–609. doi:10.1152/physrev.00024.2007
Bayeva M, Gheorghiade M, Ardehali H (2013) Mitochondria as a therapeutic target in heart failure. J Am Coll Cardiol 61(6):599–610. doi:10.1016/j.jacc.2012.08.1021
Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, Westphall MS, Pagliarini DJ, Prolla TA, Assadi-Porter F, Roy S, Denu JM, Coon JJ (2013) Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell 49(1):186–199. doi:10.1016/j.molcel.2012.10.024
Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA 105(38):14447–14452. doi:10.1073/pnas.0803790105
Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr, Alt FW, Kahn CR, Verdin E (2010) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464(7285):121–125. doi:10.1038/nature08778
Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV Jr, Weissman S, Verdin E, Schwer B (2007) Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27(24):8807–8814. doi:10.1128/MCB.01636-07
Yu W, Dittenhafer-Reed KE, Denu JM (2012) SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem 287(17):14078–14086. doi:10.1074/jbc.M112.355206
Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CF, Steegborn C (2008) Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol 382(3):790–801. doi:10.1016/j.jmb.2008.07.048
Shi T, Wang F, Stieren E, Tong Q (2005) SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem 280(14):13560–13567. doi:10.1074/jbc.M414670200
Cimen H, Han MJ, Yang Y, Tong Q, Koc H, Koc EC (2010) Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry 49(2):304–311. doi:10.1021/bi901627u
Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, van der Meer R, Nguyen P, Savage J, Owens KM, Vassilopoulos A, Ozden O, Park SH, Singh KK, Abdulkadir SA, Spitz DR, Deng CX, Gius D (2010) SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell 17(1):41–52. doi:10.1016/j.ccr.2009.11.023
Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, Olivier AK, Spitz DR, Gius D (2010) Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 40(6):893–904. doi:10.1016/j.molcel.2010.12.013
Qiu X, Brown K, Hirschey MD, Verdin E, Chen D (2010) Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12(6):662–667. doi:10.1016/j.cmet.2010.11.015
Koentges C, Pfeil K, Schnick T, Wiese S, Dahlbock R, Cimolai MC, Meyer-Steenbuck M, Cenkerova K, Hoffmann MM, Jaeger C, Odening KE, Kammerer B, Hein L, Bode C, Bugger H (2015) SIRT3 deficiency impairs mitochondrial and contractile function in the heart. Basic Res Cardiol 110(4):36. doi:10.1007/s00395-015-0493-6
Alrob OA, Sankaralingam S, Ma C, Wagg CS, Fillmore N, Jaswal JS, Sack MN, Lehner R, Gupta MP, Michelakis ED, Padwal RS, Johnstone DE, Sharma AM, Lopaschuk GD (2014) Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc Res 103(4):485–497. doi:10.1093/cvr/cvu156
Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, Samant S, Ravindra PV, Isbatan A, Gupta MP (2010) Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem 285(5):3133–3144. doi:10.1074/jbc.M109.077271
Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, Levine DC, Bacsik DJ, Gius D, Newgard CB, Goetzman E, Chandel NS, Denu JM, Mrksich M, Bass J (2013) Circadian clock NAD + cycle drives mitochondrial oxidative metabolism in mice. Science 342(6158):1243417. doi:10.1126/science.1243417
Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP (2009) Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Investig 119(9):2758–2771. doi:10.1172/JCI39162
Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA (2010) Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2(12):914–923. doi:10.18632/aging.100252
Pillai VB, Samant S, Sundaresan NR, Raghuraman H, Kim G, Bonner MY, Arbiser JL, Walker DI, Jones DP, Gius D, Gupta MP (2015) Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat Commun 6:6656. doi:10.1038/ncomms7656
Sugden PH, Clerk A (2000) Activation of the small GTP-binding protein Ras in the heart by hypertrophic agonists. Trends Cardiovasc Med 10(1):1–8
Sawyer DB, Siwik DA, Xiao L, Pimentel DR, Singh K, Colucci WS (2002) Role of oxidative stress in myocardial hypertrophy and failure. J Mol Cell Cardiol 34(4):379–388. doi:10.1006/jmcc.2002.1526
Sugden PH, Clerk A (2006) Oxidative stress and growth-regulating intracellular signaling pathways in cardiac myocytes. Antioxid Redox Signal 8(11–12):2111–2124. doi:10.1089/ars.2006.8.2111
Tan WQ, Wang K, Lv DY, Li PF (2008) Foxo3a inhibits cardiomyocyte hypertrophy through transactivating catalase. J Biol Chem 283(44):29730–29739. doi:10.1074/jbc.M805514200
Young LH (2008) AMP-activated protein kinase conducts the ischemic stress response orchestra. Circulation 117(6):832–840. doi:10.1161/CIRCULATIONAHA.107.713115
Chan AY, Dolinsky VW, Soltys CL, Viollet B, Baksh S, Light PE, Dyck JR (2008) Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J Biol Chem 283(35):24194–24201. doi:10.1074/jbc.M802869200
Meng RS, Pei ZH, Yin R, Zhang CX, Chen BL, Zhang Y, Liu D, Xu AL, Dong YG (2009) Adenosine monophosphate-activated protein kinase inhibits cardiac hypertrophy through reactivating peroxisome proliferator-activated receptor-alpha signaling pathway. Eur J Pharmacol 620(1–3):63–70. doi:10.1016/j.ejphar.2009.08.024
Ikeda Y, Sato K, Pimentel DR, Sam F, Shaw RJ, Dyck JR, Walsh K (2009) Cardiac-specific deletion of LKB1 leads to hypertrophy and dysfunction. J Biol Chem 284(51):35839–35849. doi:10.1074/jbc.M109.057273
Heineke J, Molkentin JD (2006) Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7(8):589–600. doi:10.1038/nrm1983
Opie LH, Owen P (1975) Effects of increased mechanical work by isolated perfused rat heart during production or uptake of ketone bodies. Assessment of mitochondrial oxidized to reduced free nicotinamide-adenine dinucleotide ratios and oxaloacetate concentrations. Biochem J 148(3):403–415
Pillai JB, Isbatan A, Imai S, Gupta MP (2005) Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD + depletion and reduced Sir2alpha deacetylase activity. J Biol Chem 280(52):43121–43130. doi:10.1074/jbc.M506162200
Grillon JM, Johnson KR, Kotlo K, Danziger RS (2012) Non-histone lysine acetylated proteins in heart failure. Biochim Biophys Acta 1822(2):607–614. doi:10.1016/j.bbadis.2011.11.016
Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC Jr, Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, Tian R (2013) Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab 18(2):239–250. doi:10.1016/j.cmet.2013.07.002
Porter GA, Urciuoli WR, Brookes PS, Nadtochiy SM (2014) SIRT3 deficiency exacerbates ischemia-reperfusion injury: implication for aged hearts. Am J Physiol Heart Circ Physiol 306(12):H1602–H1609. doi:10.1152/ajpheart.00027.2014
Koentges C, Pfeil K, Meyer-Steenbuck M, Lother A, Hoffmann MM, Odening KE, Hein L, Bode C, Bugger H (2016) Preserved recovery of cardiac function following ischemia-reperfusion in mice lacking SIRT3. Can J Physiol Pharmacol 94(1):72–80. doi:10.1139/cjpp-2015-0152
Bochaton T, Crola-Da-Silva C, Pillot B, Villedieu C, Ferreras L, Alam MR, Thibault H, Strina M, Gharib A, Ovize M, Baetz D (2015) Inhibition of myocardial reperfusion injury by ischemic postconditioning requires sirtuin 3-mediated deacetylation of cyclophilin D. J Mol Cell Cardiol 84:61–69. doi:10.1016/j.yjmcc.2015.03.017
Mather M, Rottenberg H (2000) Aging enhances the activation of the permeability transition pore in mitochondria. Biochem Biophys Res Commun 273(2):603–608. doi:10.1006/bbrc.2000.2994
Petrosillo G, Moro N, Paradies V, Ruggiero FM, Paradies G (2010) Increased susceptibility to Ca(2 +)-induced permeability transition and to cytochrome c release in rat heart mitochondria with aging: effect of melatonin. J Pineal Res 48(4):340–346. doi:10.1111/j.1600-079X.2010.00758.x
Hofer T, Servais S, Seo AY, Marzetti E, Hiona A, Upadhyay SJ, Wohlgemuth SE, Leeuwenburgh C (2009) Bioenergetics and permeability transition pore opening in heart subsarcolemmal and interfibrillar mitochondria: effects of aging and lifelong calorie restriction. Mech Ageing Dev 130(5):297–307. doi:10.1016/j.mad.2009.01.004
Di Lisa F, Bernardi P (2005) Mitochondrial function and myocardial aging. A critical analysis of the role of permeability transition. Cardiovasc Res 66(2):222–232. doi:10.1016/j.cardiores.2005.02.009
Fernandez-Sanz C, Ruiz-Meana M, Castellano J, Miro-Casas E, Nunez E, Inserte J, Vazquez J, Garcia-Dorado D (2015) Altered FoF1 ATP synthase and susceptibility to mitochondrial permeability transition pore during ischaemia and reperfusion in aging cardiomyocytes. Thromb Haemost 113(3):441–451. doi:10.1160/TH14-10-0901
Korzick DH, Lancaster TS (2013) Age-related differences in cardiac ischemia-reperfusion injury: effects of estrogen deficiency. Pflugers Arch 465(5):669–685. doi:10.1007/s00424-013-1255-7
Lesnefsky EJ, Gallo DS, Ye J, Whittingham TS, Lust WD (1994) Aging increases ischemia-reperfusion injury in the isolated, buffer-perfused heart. J Lab Clin Med 124(6):843–851
Sussman MA, Anversa P (2004) Myocardial aging and senescence: Where have the stem cells gone? Ann Rev Physiol 66:29–48. doi:10.1146/annurev.physiol.66.032102.140723
Karbowski M, Kurono C, Wozniak M, Ostrowski M, Teranishi M, Nishizawa Y, Usukura J, Soji T, Wakabayashi T (1999) Free radical-induced megamitochondria formation and apoptosis. Free Radic Biol Med 26(3–4):396–409
Sachs HG, Colgan JA, Lazarus ML (1977) Ultrastructure of the aging myocardium: a morphometric approach. Am J Anat 150(1):63–71. doi:10.1002/aja.1001500105
Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P (2001) Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD + and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem 276(4):2571–2575 Epub 2000 Nov 2579
Zhu J, Rebecchi MJ, Tan M, Glass PS, Brink PR, Liu L (2010) Age-associated differences in activation of Akt/GSK-3beta signaling pathways and inhibition of mitochondrial permeability transition pore opening in the rat heart. J Gerontol A Biol Sci Med Sci 65(6):611–619. doi:10.1093/gerona/glq035
Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J (2009) Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res 105(5):481–491. doi:10.1161/CIRCRESAHA.109.203703
Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J (2014) Nicotinamide mononucleotide, an intermediate of NAD + synthesis, protects the heart from ischemia and reperfusion. PLoS ONE 9(6):e98972. doi:10.1371/journal.pone.0098972
Du J, Jiang H, Lin H (2009) Investigating the ADP-ribosyltransferase activity of sirtuins with NAD analogues and 32P-NAD. Biochemistry 48(13):2878–2890. doi:10.1021/bi802093g
Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, Maechler P, Verdin E (2007) Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem 282(46):33583–33592. doi:10.1074/jbc.M705488200
Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, Wolberger C, Prolla TA, Weindruch R, Alt FW, Guarente L (2006) SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 126(5):941–954. doi:10.1016/j.cell.2006.06.057
Gertz M, Steegborn C (2016) Using mitochondrial sirtuins as drug targets: disease implications and available compounds. CMLS, Cell Mol Life Sci. doi:10.1007/s00018-016-2180-7
Laurent G, German NJ, Saha AK, de Boer VC, Davies M, Koves TR, Dephoure N, Fischer F, Boanca G, Vaitheesvaran B, Lovitch SB, Sharpe AH, Kurland IJ, Steegborn C, Gygi SP, Muoio DM, Ruderman NB, Haigis MC (2013) SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol Cell 50(5):686–698. doi:10.1016/j.molcel.2013.05.012
Nasrin N, Wu X, Fortier E, Feng Y, Bare OC, Chen S, Ren X, Wu Z, Streeper RS, Bordone L (2010) SIRT4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J Biol Chem 285(42):31995–32002. doi:10.1074/jbc.M110.124164
Laurent G, de Boer VC, Finley LW, Sweeney M, Lu H, Schug TT, Cen Y, Jeong SM, Li X, Sauve AA, Haigis MC (2013) SIRT4 represses peroxisome proliferator-activated receptor alpha activity to suppress hepatic fat oxidation. Mol Cell Biol 33(22):4552–4561. doi:10.1128/MCB.00087-13
Jeong SM, Xiao C, Finley LW, Lahusen T, Souza AL, Pierce K, Li YH, Wang X, Laurent G, German NJ, Xu X, Li C, Wang RH, Lee J, Csibi A, Cerione R, Blenis J, Clish CB, Kimmelman A, Deng CX, Haigis MC (2013) SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell 23(4):450–463. doi:10.1016/j.ccr.2013.02.024
Liu B, Che W, Xue J, Zheng C, Tang K, Zhang J, Wen J, Xu Y (2013) SIRT4 prevents hypoxia-induced apoptosis in H9c2 cardiomyoblast cells. Cell Physiol Biochem 32(3):655–662. doi:10.1159/000354469
Scarabelli TM, Gottlieb RA (2004) Functional and clinical repercussions of myocyte apoptosis in the multifaceted damage by ischemia/reperfusion injury: old and new concepts after 10 years of contributions. Cell Death Differ 11(Suppl 2):S144–S152. doi:10.1038/sj.cdd.4401544
Verma M, Shulga N (1827) Pastorino JG Sirtuin-4 modulates sensitivity to induction of the mitochondrial permeability transition pore. Biochim Biophys Acta 1:38–49. doi:10.1016/j.bbabio.2012.09.016
Nakagawa T, Lomb DJ, Haigis MC, Guarente L (2009) SIRT5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 137(3):560–570. doi:10.1016/j.cell.2009.02.026
Fischer F, Gertz M, Suenkel B, Lakshminarasimhan M, Schutkowski M, Steegborn C (2012) Sirt5 deacylation activities show differential sensitivities to nicotinamide inhibition. PLoS ONE 7(9):e45098. doi:10.1371/journal.pone.0045098
Rauh D, Fischer F, Gertz M, Lakshminarasimhan M, Bergbrede T, Aladini F, Kambach C, Becker CF, Zerweck J, Schutkowski M, Steegborn C (2013) An acetylome peptide microarray reveals specificities and deacetylation substrates for all human sirtuin isoforms. Nat Commun 4:2327. doi:10.1038/ncomms3327
Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, Luo H, Zhang Y, He W, Yang K, Zwaans BM, Tishkoff D, Ho L, Lombard D, He TC, Dai J, Verdin E, Ye Y, Zhao Y (2011) The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteom 10(12):M111-012658. doi:10.1074/mcp.M111.012658
Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y (2011) Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol 7(1):58–63. doi:10.1038/nchembio.495
Rardin MJ, He W, Nishida Y, Newman JC, Carrico C, Danielson SR, Guo A, Gut P, Sahu AK, Li B, Uppala R, Fitch M, Riiff T, Zhu L, Zhou J, Mulhern D, Stevens RD, Ilkayeva OR, Newgard CB, Jacobson MP, Hellerstein M, Goetzman ES, Gibson BW, Verdin E (2013) SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab 18(6):920–933. doi:10.1016/j.cmet.2013.11.013
Nishida Y, Rardin MJ, Carrico C, He W, Sahu AK, Gut P, Najjar R, Fitch M, Hellerstein M, Gibson BW, Verdin E (2015) SIRT5 regulates both cytosolic and mitochondrial protein malonylation with glycolysis as a major target. Mol Cell 59(2):321–332. doi:10.1016/j.molcel.2015.05.022
Sadhukhan S, Liu X, Ryu D, Nelson OD, Stupinski JA, Li Z, Chen W, Zhang S, Weiss RS, Locasale JW, Auwerx J, Lin H (2016) Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function. Proc Natl Acad Sci USA 113(16):4320–4325. doi:10.1073/pnas.1519858113
Buler M, Aatsinki SM, Izzi V, Uusimaa J, Hakkola J (2014) SIRT5 is under the control of PGC-1alpha and AMPK and is involved in regulation of mitochondrial energy metabolism. FASEB J 28(7):3225–3237. doi:10.1096/fj.13-245241
Lin ZF, Xu HB, Wang JY, Lin Q, Ruan Z, Liu FB, Jin W, Huang HH, Chen X (2013) SIRT5 desuccinylates and activates SOD1 to eliminate ROS. Biochem Biophys Res Commun 441(1):191–195. doi:10.1016/j.bbrc.2013.10.033
Boylston JA, Sun J, Chen Y, Gucek M, Sack MN, Murphy E (2015) Characterization of the cardiac succinylome and its role in ischemia-reperfusion injury. J Mol Cell Cardiol 88:73–81. doi:10.1016/j.yjmcc.2015.09.005
Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord EN, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa AS, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP (2014) Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515(7527):431–435. doi:10.1038/nature13909
Acknowledgments
This study was supported by Grants of the German Research Foundation (Bu2126/2-1 and Bu2126/3-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest related to this manuscript.
Rights and permissions
About this article
Cite this article
Bugger, H., Witt, C.N. & Bode, C. Mitochondrial sirtuins in the heart. Heart Fail Rev 21, 519–528 (2016). https://doi.org/10.1007/s10741-016-9570-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-016-9570-7