Abstract
Supplying nitrogen (N) to crops by incorporating N2-fixing green manures into soil can improve soil functioning and increase soil carbon storage. However, as with N-fertiliser use, excess mineral N from decomposed green manures can be lost via leaching as NO3− and emitted as the greenhouse gas N2O. To improve nitrogen use efficiency (NUE) we propose supplying leaf matter harvested from permanent stands of N2-fixing plants grown ex-situ as perennial mobile green manures (PMGMs), thus allowing flexible timing of additions to synchronise with N demand. To assess the effectivity of PMGM use, we monitored crop N uptake, biomass production and soil mineral N from fertilisation by three potential PMGM species, Alnus glutinosa (alder), Gunnera tinctoria (gunnera), and Ulex europaeus (gorse), compared to the conventional green manure Trifolium pratense (red clover) and NH4NO3 fertiliser. Effectivity of N fertilisation by PMGMs increased with the duration of their use. In the first season of a field experiment, crop N uptake after addition of 200 kg N ha−1 within gunnera was equivalent to that from only 14 kg NH4NO3-N ha−1, but after two yearly harvests with annual gunnera additions it increased to 70 kg NH4NO3-N ha−1 year−1 equivalent. In a 1-year pot experiment, PMGMs resulted in equal or higher crop biomass than clover-fed crops, with lower soil NO3− (≤ 25 mg N L−1 soil solution) than in clover-treated soil (≥ 130 mg N L−1). We conclude that PMGMs have potential to increase NUE over that of traditional green manures and discuss the feasibility and possible agro-ecological benefits of PMGM-fed systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Today’s high agricultural yields are made possible by supplying crops with additional plant-available N, which has been converted from atmospheric N2 by industrial N2 fixing or by the growing of N2-fixing leguminous plants (Battye et al. 2017). This has resulted in unprecedented amounts of reactive N in agricultural systems, which is environmentally detrimental regardless of the method of N2 fixation (Rockstrom et al. 2009; Smil 2011; Fowler et al. 2013). Nitrogen fertiliser production accounts for 1–2% of the world’s fossil fuel use, causing associated CO2 emissions (Houlton et al. 2019). In contrast, biological N2 fixing does not cause CO2 emissions, and addition of plant matter to soil can increase soil C storage (Lehtinen et al. 2014; McClelland et al. 2021) and improve soil health (Kibblewhite et al. 2008). Biologically fixed N2 is usually supplied via leguminous green manures such as clovers or vetches which are grown on agricultural land and incorporated in situ into soil (Blanco-Canqui et al. 2015; Carr et al. 2020). As the plant matter decomposes, N becomes available for uptake by the following crop as ammonium (NH4+), nitrate (NO3−), and small organic N molecules (Farzadfar et al. 2021; Udvardi et al. 2021). Bacterial N2 fixing, however, powered by photosynthetically fixed C, necessitates periodically setting aside cropland for sunlight energy capture, which reduces the overall yields of the cropping system (Crews and Peoples 2004; Powlson et al. 2011; Smith et al. 2018).
Nitrogen losses can occur after the addition of fertiliser or green manure N by leaching of NO3− (Neeteson and Carton 2001; Di and Cameron 2002; Campiglia et al. 2011) and dissolved organic N (van Kessel et al. 2009) and by ammonia volatilisation (de Ruijter et al. 2010; Nett et al. 2016; Pan et al. 2016). Emission of nitrous oxide (N2O) can also occur, principally by nitrification of NH4+ or denitrification of NO3− (Chen et al. 2013; Butterbach-Bahl et al. 2013; Rees et al. 2013). Global N2O emissions, the majority of which are from agricultural soils, account for 3.4% of total anthropogenic greenhouse gas (GHG) emissions, and 10% of the climate change impact of global food production (Crippa et al. 2021). Nitrogen losses increase with higher soil mineral N concentrations which occur when N supply exceeds crop demand. To reduce losses, fertilisers are commonly applied to crops in two or more (split) applications, and precision agriculture techniques are increasingly used to match supply with crop demand (Norton and Ouyang 2019; Tei et al. 2020; Rees et al. 2020). The incorporation of green manures into soil, however, offers less scope to synchronise N supply with crop demand (Cassman et al. 2002; Crews and Peoples 2005; Finney et al. 2015; Mὃller 2018).
To better match the supply of green manure N with crop demand, some growers use leaf material harvested from green manures grown outside the cropping area and added to arable land when required (Crews and Peoples 2005; Mὃller and Stinner 2009; Carter et al. 2014). For example, Burgt et al. (2018) trialled grass-legume and grass mixes, applied fresh or pelleted, and Sorensen and Thorup-Kristensen (2011) trialled various plant species as “mobile green manures” to fertilise vegetable crops. Similarly, in tropical agroforestry, soil is fertilised with green prunings from N2-fixing trees (Palm 1995; Ajayi et al. 2011; Sileshi et al. 2020).
Here we propose a system of green manuring for temperate agriculture in which N is fixed by perennials including trees and shrubs grown ex-situ in multi-species agroforestry areas, or “bio-service areas.” As far as we are aware, such a system is new to temperate agriculture, and we refer to plants used in this way as perennial mobile green manures (PMGMs). Use of species suited to growing in exposed or waterlogged conditions, allows the “bio-service areas” to be situated on marginal land, so reducing requirement for prime agricultural land for N2-fixation.
To synchronise N additions with crop demand, knowledge is needed on the rate at which N becomes available after the PMGMs are added to soil. This depends on the interaction of leaf characteristics with the physical and chemical conditions of the soil, such as pH and temperature. Nitrogen will typically be immobilised and unavailable to crops if the C:N ratio of the substrate is ≥ 25:1 (Robertson and Groffman 2007; Farzadfar et al. 2021). Over time, however, substrate C is utilised by microbes to a point where microbes become C limited and N becomes available (Whalen 2014; Chen et al. 2014; Hansen et al. 2019). Other factors of leaf chemistry such as secondary metabolites also influence N release (Trinsoutrot et al. 2000; Chomel et al. 2016). Tree leaves may contain higher concentrations of lignin or phenolic compounds than non-woody traditional green manures which may affect the speed of N release or crop growth due to allelopathic effects (Fox et al. 1990; Handayanto et al. 1997; Kumar and Goh 1999).
Use of the PMGMs as dried leaf material and addition by mulching instead of incorporation would increase ease of application and give greater flexibility in timing of additions. Mulching would also enable PMGM use in no or low-till agriculture, which can be beneficial for soil functioning (Roger-Estrade et al. 2010; Lemtiri et al. 2016) and increase C sequestration (Lal 2004; Chen et al. 2020; Cooper et al. 2021). Plant matter has been found to decompose faster when incorporated than when mulched (Coppens et al. 2007; Hoyle and Murphy 2011; Chen et al. 2014), which affects N availability and risk of N loss as NO3− and N2O (Coppens et al. 2006; Shan and Yan 2013; Nett et al. 2015). Ammonia losses are not considered to be a major factor when the material is incorporated but can be high from surface mulches (Larsson et al. 1998; de Ruijter et al. 2010).
Here, we define nitrogen use efficiency (NUE) as the overall efficiency of N use in an agricultural system, which is influenced by a variety of factors including the long-term retention of N in soil and the efficiency of plant N use (Cassman et al. 2002; Kubota et al. 2018). This is a broader concept than the nitrogen recovery efficiency (NRE), a calculation of the proportion of the N supplied taken up by treated crops above that taken up by control crops (Cassman et al. 2002; Mosier et al. 2013; Congreves et al. 2021). Another assessment of N uptake from organic amendments is the fertiliser equivalent (FE), a calculation of the amount of fertiliser N which would be required to produce the equivalent crop N uptake to that from the organic addition (Kumar and Goh 1999; Rigby and Smith 2014).
We undertook a one-year pot experiment and two-year field experiment to research the N supply to crops and risk of N pollution when fertilising crops with leaf matter added by various methods from three potential PMGM species suitable for temperate climates. Our objectives were to ascertain the rate at which N became available for crop uptake, identify any other effects of PMGM use on crop growth and quantify the mineral N concentrations in soil to assess N pollution risk. We hypothesised that PMGMs with a higher C:N ratio would have a more delayed effect on crop N uptake and soil mineral N concentrations, and that PMGMs would have an effect on N dynamics lasting more than a year after application.
Materials and methods
We investigated three species as PMGMs: Alder (Alnus glutinosa), Gorse (Ulex europaeus), and Gunnera (Gunnera tinctoria), which form associations with the N2-fixing microbes Frankia actinobacteria, rhizobia bacteria, and Nostoc cyanobacteria, respectively (Vessey et al. 2005). Alder is a deciduous tree, native to Europe and North Africa, which is tolerant of flooded ground and re-grows after coppicing (Houston Durrant et al. 2016). Gorse is adapted to exposed conditions and is invasive in some parts of the world (Cordero et al. 2016). Gunnera tinctoria is a fast-growing herbaceous perennial of wet or waterlogged soils, over 2 m tall, native to South America, but a garden plant which has become naturalised in some temperate lowland areas of Europe (NNSS 2011).
Pot experiment
Experimental design
The pot experiment monitored the crop N uptake and soil N dynamics as affected by amendments of the three PMGMs in comparison to the traditional green manure red clover (Trifolium pratense) and a range of NH4NO3 fertiliser levels. The treatments were: no addition (control), clover, alder, gunnera and gorse leaves, each containing the equivalent of 200 kg N ha−1, and NH4NO3 at the equivalent of 35 and 70 N ha−1 (referred to as: AN35 and AN70). Five replicate pots of soil with each treatment were arranged in a random block design. Each pot was sown with ryegrass (Lolium perenne) as a fast growing, reliable proxy-crop, which enabled regular harvesting to measure crop N uptake. Soil solution was regularly sampled for N analyses. The experiment was conducted in a heated greenhouse with warm, moist conditions favourable to fast organic matter breakdown to test a “worst case scenario” for excess mineral N in soil.
An additional experiment using the same methods was conducted at a later date to enable comparison of the effect of PMGMs with higher fertiliser treatments of 140 and 200 kg N ha−1 (referred to as AN140 and AN200) as described below.
Set-up and management
Soil, classified as a Eutric Cambisol (IUSS 2015), was collected from a depth of 20–120 mm from a sheep-grazed grassland at Henfaes Research Station, Abergwyngregyn, (53° 14′ N, 4° 01′ W), sieved to ≤ 10 mm and stored at 4 °C for 18 days until the experiment began. Soil characteristics are shown in Suppl Table S1. P, K, S and Ca additions were made to soil as appropriate before addition of treatments, to ensure that crop growth was not limited by an inappropriate pH, or lack of nutrients other than N. Additions (Suppl Table S2) were based on analyses by NRM laboratories (Cawood scientific, Bracknell, UK) and recommendations by UK fertiliser guidelines (Defra 2010; AHDB 2018).
The green manures were collected and stored as described in the supplementary information (SI), with characteristics described in Suppl Table S3. Soil and treatments were added to plastic pots 272 mm high by 113 mm by 80 mm (internal) without drainage holes. Each pot contained fresh soil weighing 1216 g, giving a total weight of soil plus pot of 1400 g each. Prior to pot filling, the fresh green manures were cut to pieces of ≤ 40 mm and thoroughly mixed with soil, except for 20 mm of soil in the uppermost layer to allow for even seed germination. NH4NO3 was added in solution, and the same volume of distilled water added to all replicates not receiving NH4NO3. Each pot was sown with 1 g ryegrass seed. Water-filled pore space (WFPS) was maintained at 70 ± 10% throughout the experiment and the greenhouse was heated and lit for 12 h daily. Mean air temperatures for each growing period ranged from 17.5 to 28.8 °C (Suppl Table S4) as measured by a sensor placed between the pots.
Soil solution was sampled throughout using 50 mm Rhizon™ soil solution samplers (male Luer 19.21.22, Rhizosphere research products, Wageningen, Netherlands) as described in SI. After 31 days, all grass in each pot was cut at a height of 10 mm above the soil surface and retained. This was repeated after a further 28 days, then at 35-day periods until the tenth cut on day 340. On day 364, all vegetation above the soil surface was removed and the soil of each pot was cut vertically into two equal blocks. Roots within one block were washed from soil and retained. Soil from the remaining block was retained in polythene bags at 4 °C prior to analyses. All plant matter was dried at 80 °C and stored until analyses.
The additional experiment included the control and AN70 treatments alongside the two higher fertiliser treatments, of AN140 and AN200, and followed the same methods as the main experiment. See SI for further details and methods of statistical validation for use of AN140 and AN200 data alongside that of the main experiment.
Field experiment
Experimental design
The 2-year experiment assessed crop N fertilisation and N dynamics in field conditions using 4 replicates of plots treated with PMGM leaf material alongside a control (no treatment) and a range of NH4NO3 fertiliser levels. The treatments were arranged in a randomized block design in Year 1, with an additional factor added as a split-plot in Year 2. Prior to treatment addition in both years, soil was prepared and amended to prevent deficiencies of nutrients other than N as described in SI. Soil cores were taken throughout to monitor soil N, and above ground vegetation sampled by quadrats as described below.
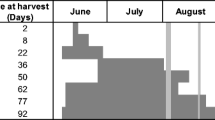
In Year 1 the treatments (Suppl Table S7) were alder, gorse and gunnera containing 200 kg N ha−1 equivalent and NH4NO3 of 25, 50, 100 and 200 kg N ha−1 equivalent referred to as AN25, AN50, AN100 and AN200, and no addition (control). To assess whether drying the PMGMs or adding as mulch affects N dynamics, alder was added in four different ways: fresh and incorporated (Ald F Inc); fresh mulched (Ald F M); dried incorporated (Ald D Inc) and dried mulched (Ald D M). Incorporation was to a depth of 100 mm. Gunnera and gorse were applied dried and mulched (Gun D M and Gorse D M). The fast-growing, reliable proxy-crop phacelia (Phacelia tanacetifolia) was sown due to its reliability against pests and diseases.
In Year 2 the experiment continued on selected plots using a split-plot design in which treatments were repeated on one half of each plot with no treatment added on the other half. This enabled assessment of the legacy effects of the original applications on Year 2 crops and soil, and the cumulative effects of two applications. The control, all AN treatments, Ald D M and Gun D M were continued on one half on each plot, referred to as control, AN 25 + 25, AN 50 + 50, AN 100 + 100, AN 200 + 200, Ald + Ald and Gun + Gun. This treatment selection was made on the basis of PMGM results in the pot experiment and Year 1 of the field experiment. The other half of the AN200, Ald DM and Gun DM treated-plots were monitored, while receiving no further treatment and referred to as AN 200 + 0, Ald + 0 and Gun + 0 (Suppl Table S7). Plots were sown with spring wheat (Triticum aestivum) ‘Mulika’ to trial a common agricultural crop.
Site and materials
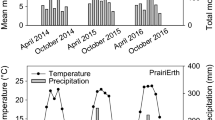
The experiment was conducted at Henfaes Research Station, Abergwyngregyn, (53° 14′ N, 4° 01′ W), on previously sheep-grazed grassland, of the same soil type as the pot experiment (Suppl Table S1), 10 m above sea level, with a temperate climate and average annual rainfall of 1060 mm. Mean daily ground-level temperatures ranged from 13–25 °C, and 11–22 °C in Year 1 and Year 2 respectively (Suppl Tables S6a/b) as recorded hourly by automatic sensors located at air and ground levels approximately 100 m from the experimental plot. Rainfall (recorded at the same location as temperature) was atypically low in both years totalling 16.4 mm and 83.5 mm during the crop growing periods of Year 1 and Year 2 respectively (Suppl Tables S6a/b). PMGM material was collected and processed as described in SI.
Year 1 experimental set-up and management
The sowing date was 21st June 2017 due to the need to collect, process and analyse the green manures from the current season’s growth. Treatments were added to plots of 1.2 by 1.3 m, separated by 0.5 m buffer strips covered with woven plastic fabric. The C:N ratios were 19:1, 12:1, 15:1 and 20:1 of fresh alder, dried alder, gunnera and gorse respectively (Suppl Table S3). Phacelia was sown at 6.7 g m−2, by a mechanical drill in rows spaced 130 mm apart.
Forty days after sowing, above-ground biomass was sampled within a quadrat of 300 by 250 mm by cutting to within 20 mm of the soil surface, and vegetation outside of the quadrats was also removed. Phacelia was re-sown by hand, at the same rate, and sampled again by quadrats after a further 65 days. The second crop contained (non-leguminous) weeds averaging 27% of the biomass dry weight (compared to < 1% weed content in the first crop), which were processed and quantified, and data included as crop production along with Phacelia. Soil cores to 100 mm depth were taken throughout the experiment, no closer to any plot edge than 200 mm, and from depths of 0–150 mm and 150–300 mm the following winter, (day 209), and extracts prepared for analyses as described below.
Year 2 experimental set up and management
Collection and drying of PMGMs in the previous summer allowed an earlier sowing date than in Year 1 of 27th April 2018. Plots, which had been left with Phacelia stubble over winter, were divided into half-plots of 1.2 m by 0.65 m and randomly assigned to receive or not receive the repeated treatment. Treatments were added immediately before sowing by the methods used in Year 1 for dry mulched PMGMs. The C:N ratios of the alder and gunnera were 17:1 and 22:1 respectively (Suppl Table S3). Wheat was hand sown at 200 kg ha−1 (9 rows per plot, 130 mm spacings). Actions to exclude pests (rodents and birds) are described in SI. Soil cores were taken to 100 mm depth throughout the experiment, and extracts prepared for analyses as described below. Ammonia emissions from soil were also monitored by trapping NH3 in H2SO4 solution as described in SI but this was only partially successful.
At 14-day intervals the wheat life cycle stages were noted as described in AHDB (2016), and chlorophyll meter readings taken (SPAD-502Plus Marunouchi, Chiyoda, Tokyo, Japan) as an indication of leaf N content. Readings were taken halfway between the tip and base of the uppermost fully expanded leaf as recommended by Debaeke et al. (2006), from 10 randomly selected plants. Biomass was sampled earlier than planned on day 111 (16/08/18), when grain was almost ripe (growth stages GS87 to GS91), due to signs of damage by rodents (SI). Samples were taken within quadrats (200 by 400 mm) not within 250 mm of any adjacent treatment or within 200 mm of the buffer strip. Grain was extracted from the ears, separated from chaff by winnowing and stems and chaff were combined for analyses. Soil cores of 80 mm diameter and to a depth 0–150 mm and 150–300 mm were taken on day 130–sample roots which were washed from soil. Over 70% of wheat root biomass is typically found in the top 300 mm of soil (ADHB 2016).
Analytical methods (pot and field experiments)
Plant material of the green manures used and harvested crop biomass was analysed as follows. Samples were dried at 80 °C for 48 h and ground to ≤ 1 mm, using a FOSS CT 193 Cyclotec mill (Hilleroed, Denmark) or a Retch mixer mill MM 200 (Haan, Germany) as appropriate for sample size and texture. Biomass was analysed for total C and N content using a TruSpecR analyser (Leco Corp., St Joseph, MI). Pre and post-drying weights were used to calculate moisture content as necessary to calculate N content of fresh green manures.
Samples of the original soil from both experiments were dried and analysed for total C and N as for biomass. Fresh soil from both experiments was analysed for pH in water at a ratio of fresh soil:deionised water of 1:2.5. Extracts of fresh soil from the field experiment were prepared using a 6 g subsample in 25 ml 0.5 M K2SO4, shaken for 60 min at 200 rev min−1, and centrifuged for 10 min at 2000 g. Soil water content of each sample was determined by weight before and after drying at 105 °C for 24 h. The supernatant was filtered and stored at − 20 °C until analysis. Field soil extracts and Rhizon-extracted pot experiment soil solution were analysed for total dissolved nitrogen (TDN) using a Multi N/C 2100S analyser (AnalytikJena, Jena, Germany), and for NO3− using a PowerWave XS BioTek spectrophotometer (Winooski, VT, US) using Gen 5 software, following the methods described in Miranda et al. (2001) for NO3−. For extractions, the above were converted to mg kg−1 dry soil. Pot experiment soil solution was analysed for total phenolics at wavelength 725 nm using Folin–Ciocalteu reagent (Swain and Hillis 1959).
Calculations and statistical analyses
To enable comparison of N uptake between the pot experiment and both years of the field experiment in different temperatures, cumulative degree days (CDD) were calculated using the total of the mean daily ground temperatures above 5 °C as in Bhogal et al. (2016). The FEs of N uptake were calculated by use of linear regressions of crop response to NH4NO3 as in Sánchez-Rodríguez et al (2018). To ensure that the fertiliser response range was linear, the NH4NO3 application rates used for field experiment Year 1 were 0, 25 and 50 kg ha−1 AN-N. Fertiliser equivalents for alder and gunnera of the cumulative effect of both years of the field trial were calculated using the total above-ground N uptake over both years against additions of NH4NO3–N in both years. Relationships between green manure C:N ratios and shoot N uptake, and soil TDN concentrations and shoot N uptake were also analysed using linear regression.
One-way ANOVAs were conducted with the treatment as a fixed factor to determine differences in N uptake and biomass production within time periods and measures which applied to the whole of each experiment, for example whole plant biomass and root:shoot ratio.
Two-way ANOVAs with treatments as the between-subjects fixed factor and time points as the within-subjects fixed factor were conducted for soil concentrations of NO3− across both experiments, and phenols and TDN in the pot experiment.
To compare the effects of the treatments on N uptake in the field over a single season (Year 1) with the cumulative effect of two years of the field experiment, we used paired t-tests between the FEs of alder and gunnera treatments of Year 1 and those over both years. In addition, a two-way ANOVA was used to analyse the grain weight produced in Year 2, with fixed effects being the type of N added (AN, alder or gunnera) and whether N was added in Year 1 only or in both years.
Before analyses, data were tested for homogeneity and normality using Levene’s test and the Shapiro Wilk test, respectively. Pot experiment and field experiment Year 2 NO3− and pot experiment TDN data required transformation before analyses to meet the requirements. Transformation was by log 10 (data + 1), the addition of a constant necessary due to some zero values. Transformed data sets are included in SI. Tukey’s HSD tests were conducted to determine significant differences between treatments. Differences between sampling time points were determined using post hoc analysis with a Bonferroni adjustment. Where comparisons were required within two-way ANOVAs between treatments at the same sampling time point, or between sampling time points within a treatment group, P values were obtained by use of the general linear model univariate procedure and by use of the split-file procedure respectively. All statistical analyses were performed using SPSS v25 (IBM Corp., Armonk, NY).
Results
Pot experiment
Crop N uptake
The clover and AN140/200 resulted in fast uptake of N and a high grass shoot N content of over 5% (Suppl Table S8a) within the first 31 days. Uptake of PMGM-N into shoots was initially low (Fig. 1, Suppl Table S8b), but by day 234 N uptake from all three PMGMs was equal to that from AN70. The C:N ratio of the green manures affected initial N uptake rates, but was not wholly accountable for them, with a regression between the C:N ratios and shoot N uptake after 31 days showing a negative relationship (y = − 0.183x + 7.2944, P = 0.006 R2 = 0.35). The plot of N uptake into grass shoots against soil TDN during the first 31 days showed a clear separation of the data points with one cluster being the clover, AN35 and AN70, and the other of PMGM treatments (Fig. 2). Separate regressions of each cluster showed that N uptake from PMGM-treated soil increased more per unit of soil TDN than that of the N uptake from the clover and AN-treated soil in which TDN was higher. The gunnera treatment resulted in a large amount of N within root tissue, > 20% of the whole plant N, so that the total plant N of gunnera-treated grass at the end of the experiment was equal to that of clover-treated grass (Table 1).
Pot experiment cumulative N content of successive harvests of above-ground grass biomass. Treatments were no addition (control), 200 kg N ha−1 equivalent in clover, alder, gunnera and gorse leaf tissue, and NH4NO3 (AN) from 70 to 200 kg N ha−1 as stated in the key. These data are also listed in Suppl Table S8b with letters denoting differences between treatments at P < 0.05. N contents on day 31 for AN 140 and 200 are not included due to differences in control and AN70 data between the main and additional experiment (Suppl Table S5a). Means ± SEM (n = 5)
Pot experiment biomass N uptake in relation to the weighted mean of TDN in soil solution during the first 31 days after addition (Suppl Table S11). Treatments were 200 kg N ha−1 equivalent in clover, alder, gunnera and gorse leaf tissue, and 35 and 70 kg N ha−1 equivalent in NH4NO3 (AN) as stated in the key. Linear trendlines of PMGMs (P = 0.015) and clover/AN35/AN70 (P = 0.01)
Biomass production
The shoot biomass production of AN200-treated grass peaked between 31 and 59 days, and that of clover, alder and gorse-fed grass peaked between 59 and 94 days (Suppl Table S9a/b). Shoot production by gunnera-fed grass, however, did not peak until 94–129 days and remained higher than from clover or AN-treated grass until day 269 (P < 0.05). The total shoot, root and whole plant biomass dry weights at the end of the experiment are shown in Table 1. Gunnera-treated grass had a higher root:shoot ratio than the control, alder, clover or AN200-treated grass, and higher root, shoot and whole plant biomass production than all other treatments. Alder and gorse treatments resulted in equal root, shoot and whole plant biomass to that of clover and AN200-fed plants.
Soil chemistry
Some measurements of N in solution for the control and AN70 differed between the main and additional experiment, and therefore data are presented from the main experiment only. The clover and AN treatments resulted in high NO3− in soil solution in the first few weeks, whereas those from PMGM treatments remained low (Fig. 4a; Suppl Table S10a/b/c). There was a significant interaction between time and treatment for soil NO3− concentrations (P < 0.001). All treatments, including the control, resulted in higher NO3− concentrations on one or more days up to and including day 5 than on all days from day 67 onwards (P < 0.05). This trend was much more pronounced for the AN70 and clover treatments with NO3− in soil solution peaking at 158 ± 10 mg L−1 on day 5 and 131 ± 18 mg L−1 on day 32 respectively, whereas that from PMGM-treated soil remained below 25 mg N L−1 throughout. NO3− in PMGM-treated soil was lower than in AN70-treated soil from days 1–25 inclusive (P < 0.05) and lower than in clover-treated soil on days 3–53 inclusive (P < 0.05). Gorse-treated soil had higher NO3− concentrations than the control on days 9, 13 and 18 but alder- and gunnera-treated soil NO3− was no higher than the control at any point.
Total dissolved nitrogen in soil solution closely followed the same treands as NO3−, and many data points for TDN concentrations were little or no higher than NO3− (Suppl Table S11a/b/c/d). Clover and AN70 treatments peaked at over 140 mg L−1 on day 32 and 156 mg L−1 on day 5 respectively, whereas gorse, alder and gunnera-treated soil TDN remained below 36, 15 and 18 mg N L−1 respectively. AN35 and AN70 resulted in higher TDN than all PMGMs up to and including day 18 (P < 0.05) and clover-treated soil TDN was higher than that of PMGMs up to day 53 (P < 0.05). There was a significant interaction effect between time and treatment (P < 0.001). All treatments, including the control, showed a decline over time with days 95 and after having lower TDN than some days before and including 39 (P < 0.05). In the latter part of the experiment, however, there was a trend of increased TDN in gorse-treated soil, compared to other treatments; for example on days 95 and 151 gorse-treated soil TDN was slightly, but significantly higher than that of any other treatment (P < 0.05).
The two-way ANOVA for phenolic compounds showed an interaction effect between treatment and time (P < 0.001). Gorse-treated soil solution had higher concentrations of phenolics over the course of the experiment than that of all other treatments (P < 0.05), and concentrations in gorse-treated soil were lower on day 318 than on day 263 (P = 0.01; Suppl Table S12a/b/c). Clover, alder and gunnera treated soil also had higher phenol concentrations than the control over the course of the experiment (P < 0.05), but no significant differences to the control on any single sampling day.
Field experiment
Crop N uptake
As in the pot experiment, a regression of C:N ratio against crop N uptake after 40 days in Year 1 showed a negative relationship (y = − 0.3086x + 12.379, P = 0.01 R2 = 0.50). The method of application of alder as either fresh or dry, mulched or incorporated material did not show an effect on N uptake (Table 2). In Year 2, the chlorophyll meter readings were lower in Gun + Gun-treated plants on day 26 than the control (P = 0.016), but there were no differences from day 40 onwards (Suppl Fig S1).
There were similar trends to that of the pot experiment of low initial N uptake from PMGMs which increased over time relative to that from AN treatments. In Year 2, the N uptake of Ald + 0 and Gun + 0 treatments did not differ from that of the control; therefore, no legacy effect from the Year 1 additions on Year 2 N uptake was evident. However, there were trends in the data that showed that the repeated additions of alder and gunnera increased N uptake compared to one addition alone. The repeated gunnera applications in Year 2 resulted in a greater N uptake FE over the two years (70 kg NH4NO3 N ha−1) than from one application in Year 1 (14 kg NH4NO3 N ha−1), significant at the 0.1% probability level (P = 0.065). The PMGM FEs of the field experiment in relation to CDDs, compared to the first three time periods of the pot experiment, are shown in Fig. 3.
A comparison of the fertiliser equivalents (FEs) of the above-ground biomass N uptake in the pot (PotExp) and field experiments Year 1 (FieldExp1) and Year 2 (FieldExp2) plotted against cumulative day degrees (CDDs). FEs represent the amount of NH4NO3 N required to produce the equivalent N uptake into the crop biomass as the addition of the 200 kg ha−1 N supplied as the PMGMs. *Combined Year 1 + 2 field experiment FEs are the amount of NH4NO3 N required per year, to result in the equivalent N uptake from the PMGM treatments per year. As the Year 1 + 2 treatments comprised two additions at different times, CDDs are not applicable. Field experiment data are means ± SEM (n = 4). Pot experiment data are means ± SEM (n = 5)
Biomass production
In Year 1, the crop 1 and total (crops 1 + 2) biomass produced by the PMGM-fed plants was no different from that of the control and the method of application of alder showed no effect (Table 2). Biomass of PMGM-treated plants in crop 2, however, did show some increases above the control and all PMGM treatments other than gorse produced crop 2 biomass equal to that of AN200-treated plants. Year 2 grain weights ranged between 5.1 and 7.4 tonnes ha−1 equivalent (Table 3). There were no differences between the individual treatments in the above-ground biomass dry weights. However, a two-way ANOVA between the quantity of N added and the type of N added showed a higher grain weight with addition of 200 kg ha−1 N in both years compared to treatments with N added in Year 1 only (P = 0.037) but no difference for the type of N added or interaction effect.
Soil chemistry
Though the effects from PMGMs were much less pronounced in the field, soil NO3− followed similar trends to the pot experiment in both years (Fig. 4). PMGM-treated soil NO3− concentrations remained no different from that of the control throughout both years of the field experiment. There was a significant interaction between time and treatment in both years (P < 0.001). The AN200 treated soil in Year 1, and the AN100 + 100 and AN200 + 200 treated soil in Year 2 had higher NO3− concentrations than the control (P < 0.001; Suppl Tables S13/14). In both years, some later sampling dates had lower soil NO3− concentrations than some earlier sampling dates (Suppl Table S13/14).
Soil NO3− N concentrations in the first 70 days after N additions of 200 kg N ha−1 equivalent within leaf tissue (clover, alder, gunnera or gorse) or NH4NO3 (AN) at the levels stated in the key; a Pot experiment in soil solution, means ± SEM (n = 5); b, c Field experiment Year 1 and field experiment Year 2 respectively, concentrations in dry soil at 0 to 100 mm depth, means ± SEM (n = 4). The treatments in field experiment Year 2 refer to those added in the previous year only, or in both the previous and current years. P values of significant differences between treatments and sampling time points are included in Suppl Tables S10/13/14
AN treatments produced higher soil TDN concentrations than from PMGMs within the first few weeks of both years (Suppl Table S15). There were no differences in TDN after day 41 in either year, or between the upper 150 mm and 150–300 mm soil cores on day 209. There was a trend of increased pH in the gunnera-treated field trial soil, with the Gun + Gun and Gun + 0 treated soil having a pH of 6.7 and 6.8 respectively at the end of Year 2, which was higher than that of the AN200 + 200 treated soil at 5.9 (P < 0.05) (Suppl Table S16).
Potential ammonia volatilisation
Where measurement of NH3 volatilisation was successful, during the first 26 days of Year 2, volatilisation was low from all treatments at < 0.01% of N supplied.
Discussion
PMGMs provided effective fertilisation with low risk of N pollution
The biomass production and crop N uptake in the pot experiment showed effective N fertilisation by PMGMs compared to that by clover, while soil NO3− concentrations remained low. The low concentrations of mineral N in PMGM-treated soil do not necessarily indicate that N availability limited plant growth, as both N release and plant uptake can be rapid and may be in balance (Jackson et al. 2008). High N uptake from gunnera-treated soil from day 129–269 indicates good synchrony of N availability with crop demand. In contrast, the initial high N content in clover and AN70/140/200-treated grass shoots and high NO3− and TDN in soil solution demonstrated that plant available N was exceeding crop demand. Excess soil mineral N was at risk to losses as N gasses including N2O, and if drainage had not been prevented would have been prone to leaching (Cameron et al. 2013; Hansen et al. 2019).
The warm moist, conditions of the pot experiment may occur in protected, irrigated cropping, in the field in tropical regions, and increasingly in temperate regions as the climate becomes more unpredictable. It should be noted that clover grown and incorporated in situ would include root tissue, and therefore may have a higher C:N ratio, slower decomposition rate and result in less excess mineral N (Li et al. 2020). Even so, these results highlight the need to consider N pollution when using green manures. Though here, clover added in this quantity proved a pollution risk, clover could also be used effectively as a PMGM, by adding smaller quantities at times of high demand.
Though the differences between treatments in the field experiment were less pronounced than in the pot experiment, the N uptake and NO3− concentrations followed the same trends, suggesting that PMGM-N may also be less at risk to losses in the field compared to conventional fertilisation. Differences in crop yield and N uptake between treatments in the field experiment are likely to have been reduced by the dry conditions, limiting both PMGM decomposition (De Neve and Hofman 2002; Whalen 2014) and crop growth (Dodd et al. 2011), Crop growth was possibly also restricted by lower than optimum pH, despite Calcifert additions. The wheat yields of 5.1–7.8 tonnes grain ha−1, however, were within the expected range, with an average UK wheat yield of 7.8 tonnes ha−1 in 2018 (Defra 2019). It is also possible that with a greater rooting area in the field than in the pot experiment, N availability was less of a limiting factor.
Factors other than C:N ratio also influenced N dynamics and crop growth
Low initial N uptake by gunnera-treated plants across the experiments suggests that gunnera had an immobilisation effect on N. As the gunnera did not have a particularly high C:N ratio, this is likely due to other aspects of gunnera leaf chemistry. After the first few weeks of the pot experiment, when measured NO3− concentrations fell to zero, TDN was a useful measure of N which may be or soon become available for plant uptake. There was low N uptake from gorse-treated soil despite higher or equal TDN concentrations. There may have been an allelopathic effect from gorse leaves, which is consistent with the higher concentrations of phenolics in the gorse-treated soil solution, which have been known to have allelopathic effects (Batish et al. 2008; Chomel et al. 2016). Emissions from gorse branches have been shown to inhibit root growth in seedlings of Amaranthus retroflexus and Digitaria Sanguinalis (Pardo-Muras et al. 2018). Hence gorse was discontinued in the field in Year 2. However, Pardo-Muras et al. (2020) showed that the allelopthathic effect of gorse may be species selective and therefore in specific circumstances it is possible that gorse could be used as a PMGM while also having weed suppressing qualities.
The higher root mass and root:shoot ratio of gunnera-treated plants over that of the control and AN-treated plants in the pot experiment may have been a response to availability of organic N (Cambui et al. 2011; Lonhienne et al. 2014; Franklin et al. 2017), or to an uneven distribution of organic matter (Robinson 1994; Hodge et al. 1999). However, these possible causes do not explain gunnera producing higher crop root growth than that from the other green manure treatments, and therefore the cause remains unknown.
The lack of differences in N uptake or crop yields between the fresh or dried and mulched or incorporated alder in Year 1 of the field experiment indicated that mulching of dried material would have no adverse effect, and was therefore the sole method used in Year 2. Differences between the fresh and dried alder, however, may have been negated by the lower C:N ratio of the dried leaves, and a larger surface area for microbial access as the dried material easily crumbled (Nicolardot et al. 2001; Moorhead and Sinsabaugh 2006; Lehtinen et al. 2014). Rainfall dampened the mulched material which was soon shaded by the crop which likely preserved moisture. Therefore, in other field conditions, differences may occur and more experimentation is required.
PMGMs had a delayed fertilisation effect
In addition to the slower crop uptake of N from PMGMs to that from clover and fertiliser over the year of the pot experiment, the increase in N uptake after gunnera application in the field over both years compared to one year only indicates that the cumulative effect of applications was to increase plant available N. Gunnera may therefore be increasingly effective at fertilising crops after further repeated applications. This could be due to increased N release because of a “home field advantage” effect where amendments decompose faster in soil that has received the same input in the past (Austin et al. 2014; Palozzi and Lindo 2018). Alternatively, stabilised gunnera from the previous year’s treatment may have been stimulated to decompose due to a priming effect of the second addition (Fontaine et al. 2003; Di Lonardo et al. 2017).
Potential to tailor PMGM use to maximise NUE
The rates at which N became available from gunnera and alder could be appropriate for long-season annual vegetable crops that have a low initial N demand (Hartz 2007; Rahn 2012). Different PMGMs could be added at different points in the cropping season as appropriate; e.g. clover could be used as a PMGM when crops are growing quickly and a fast N release rate is desirable. Strategically combining different PMGMs and other amendments could enable tailoring of N release to the crop (Handayanto et al. 1997; Gunnarsson and Marstorp 2002; Kaewpradit et al. 2008; Truong and Marschner 2018; Rothe et al. 2019). For example, gunnera could be used to temporarily immobilise excess N if added alongside fertiliser or N-rich crop residues until the crop (or cover crop) exerts an adequate N demand (Congreves and Eerd 2015). To maximise NUE, precision agriculture techniques as being developed for fertiliser use could be used to target PMGM application.
The higher root mass in gunnera-treated plants in the pot experiment may have had the effect of increasing N uptake, both directly by roots and via associated microbes (Jackson et al. 2008; Moreau et al. 2019). Higher root mass can also increase N availability by stimulating further decomposition of gunnera or other soil organic matter (Clarholm 1985; Zhu et al. 2014; Farzadfar et al. 2021). Therefore, if higher root mass is shown by further experimentation to be a repeatable effect, this could be useful in appropriate crops, such a long-lived crops in low-input systems.
Some production systems may be more suited to PMGM use, for example, polytunnel vegetable production where cropping space is at a premium and risk of N loss is high due to warm temperatures and high moisture. Large-scale arable farming areas where animal manure is in short supply could benefit from PMGM organic matter to improve soil structure, especially in sandy soils and heavy clays. This research considered the effect on N fertilisation only, however other nutrients such as phosphorus and potassium may also be present within PMGMs in useful quantities. Therefore, PMGM use could reduce other external inputs, as could the effect of gunnera on increasing pH where this is desirable.
Other N2-fixing perennials could also be used as PMGMs, and knowledge of the N release rates from leaves of other species would increase scope for improving synchrony. Production of a PMGM database for temperate plants, similar to that developed for tropical species by Palm et al. (2001), listing characteristics such as C:N ratio, P and K contents, and phenolics would be valuable for future PMGM selection.
NUE in relation to organic N
Supply of N within organic material gives opportunity for uptake of intact organic N which negates the need for mineralisation and therefore bypasses the stages where N is most prone to loss (Paungfoo-Lonhienne et al. 2012; Franklin et al. 2017). Organic N uptake is increasingly recognised in crop nutrition (Hill et al. 2011; Ma et al. 2018; Moreau et al. 2019) and offers a further advantage of increasing NUE due to a C bonus to the plant and lower N processing costs (Schmidt et al. 2013; Franklin et al. 2017; Farzadfar et al. 2021). In addition, crop breeding that selects for productivity in a high mineral N environment, may have reduced the capacity for organic N uptake in modern crop varieties (Finlay and Wilkinson 1963; Hirel et al. 2011). Restoring affinity for organic N uptake into new crop varieties could tighten the N cycle increasing NUE of green manure fed systems (Paungfoo-Lonhienne et al. 2012; Bardhan and Patel 2016; Congreves et al. 2021; Farzadfar et al. 2021). Although it is unknown what form of N was taken up from our PMGM treatments, the scope for improving organic N uptake, alongside targeted addition by PMGMs, could prove a valuable research avenue.
NUE in a wider temporal and spatial context
Despite intensive research into increasing NUE in arable crops, there has been little progress in recent times (Bowles et al. 2018; Farzadfar et al. 2021). Typically, less than 50% of the N supplied is found be taken up by crops in the same year (Udvardi et al. 2021). Scope for increasing NUE by the recommended “4Rs” for N application (right rate, time, source and place) (Udvardi et al. 2021) is likely to be futher limited by a changing climate, with increasingly unpredictable N release and crop demand (Bowles et al. 2018; Zhang et al 2021). Additions of N in the current year, however, may contribute less to N uptake than previously thought (Yan et al. 2020), with a large proportion of N uptake being from stabilised soil N (Sebilo et al. 2013; Lupwayi and Soon 2015; Celestina et al. 2019; Pullens et al. 2021). Yan et al. (2020) proposed instead that N applications be considered as replenishing N reserves for crop fertilisation in future years, reminiscent of the old organic adage “feed the soil, not the plant” (Friedel and Ardakani 2021). If the trends seen in our results of increasing N uptake with longer periods of PMGM use are found to continue over the long term, this may further increase NUE. In addition, it is increasingly recommended that more perennials be included in the agricultural landscape to intercept leached N and reduce loss by wind and water erosion (Lin et al. 2016; Diederich et al. 2019; Bowles et al. 2018). It may be advisable, therefore, to accept that a high NUE within each field and each year may not be achievable, and instead aim for N retention on a greater spatial and temporal scale (Congreves et al. 2021). The integration of PMGM production into farmland could help with these aims, as discussed below.
PMGM production in the agricultural landscape
Integrating PMGM production within agricultural landscapes would provide various co-benefits, including habitat provision for the biodiversity necessary for agricultural functioning (Pilling et al. 2020) and flood and erosion prevention by slowing of water flow by tree roots (Marshall et al. 2014; Caulfield et al. 2020). Replacement of rotational green manure leys with permanent unploughed areas of PMGMs is likely to increase soil C sequestration (Lal 2004; Lamb et al. 2016; Balmford et al. 2018), though arable land under yearly copping, without leys, must still be managed to maintain soil C by use of winter cover crops alongside the addition of the PMGMs. PMGMs could be grown in multi-species “bio-service areas” for example, short rotation alder coppice, with understories of N2-fixing perennials such as clovers and vetches, as well as non N2-fixers such as comfrey (Symphytum), the inclusion of which helps regulate excess N in soil and reduce losses (De Notaris et al. 2021; Fig. 5).
Care must be taken on choice of species for appropriate areas as, for example, Gunnera can be invasive and its planting is now banned in the UK (Defra 2022); however, where gunnera clearance is needed for habitat conservation, the use of the waste vegetation as a PMGM may be possible. PMGM production areas could be integrated with other landscape features such as restored or widened hedgerows and buffer strips to protect watercourses from leached N (Di and Cameron 2002). Here, deep-rooted plants could capture leached N to be returned to cropland within the PMGM tissue (Bowles et al. 2018). PMGM production may also be an economically useful alternative land use to ease transition to a lower dependence on ruminant production, as necessary for climate change mitigation (Loboguerrero et al. 2019; Willett et al. 2019; Hayek et al. 2021), along with a mobile source of N to replace animal manure.
Conclusions
Perennial mobile green manures (PMGMs) were effective at supplying N to crops and resulted in equal or greater crop biomass production than clover-fed crops in a pot experiment, with a lower risk of N pollution. Effectivity as a fertiliser was greater over longer time periods and data from a 2-year field experiment indicated that repeated PMGM use may be increasingly effective. Gorse was deemed the least suitable due to a possible allelopathic effect. This knowledge of N uptake rates could enable the matching of alder and gunnera, and also clover, applied as PMGMs for N fertilisation with suitable crops. This system has the potential to increase nitrogen use efficiency (NUE) over that achieved by traditional green manures, while retaining the advantages of biologically fixed N2 of organic matter addition and negating CO2 emissions. An increased understanding of N dynamics, including organic N uptake and N conservation over a greater spatial and temporal scale, could enable improvements in tailoring PMGM use for NUE. Integration of PMGM production into agricultural landscapes could bring further agro-ecological co-benefits.
Collating and increasing knowledge of characteristics of a wider range of potential PMGM species would give greater scope for the technique, and the practicalities of PMGM use requires further experimentation. Research is also needed on the wider climate change impact both within the cropping areas and within the PMGM production areas, such as by quantification of soil C storage, and of greenhouse gas emissions from harvesting and processing.
Data availability
Any data generated or analysed during this study which are not included in this published article or its supplementary information files are available on request from the corresponding author.
References
AHDB (Agriculture and Horticulture Development Board, Stoneleigh Park, Warwickshire) (2016) Wheat growth guide. Accessed at https://cereals.ahdb.or.uk/media/185687/g66-wheat-growth-guide.pdf. Accessed on 16 Mar 2018
AHDB (Agriculture and Horticulture Development Board, Stoneleigh Park, Warwickshire) (2018) Nutrient management guide (RB209) Section 4 Arable Crops. Accessed at https://media.ahdb.org.uk/media/Default/Imported%20Publication%20Docs/RB209%20Arable%20crops.pdf
Ajayi OC, Place F, Akinnifesi FK, Sileshi GW (2011) Agricultural success from Africa: the case of fertilizer tree systems in southern Africa (Malawi, Tanzania, Mozambique, Zambia and Zimbabwe). Int J Agric Sustain 9(1):129–136. https://doi.org/10.3763/ijas.2010.0554
Austin AT, Vivanco L, Gonzalez-Arzac A, Perez LI (2014) There’s no place like home? An exploration of the mechanisms behind plant litter-decomposer affinity in terrestrial ecosystems. New Phytol 204(2):307–314. https://doi.org/10.1111/nph.12959
Balmford A, Amano T, Bartlett H, Chadwick D, Collins A, Edwards D, Field R, Garnsworthy P, Green R, Smith P, Waters H, Whitmore A, Broom DM, Chara J, Finch T, Garnett E, Gathorne-Hardy A, Hernandez-Medrano J, Herrero M, Hua F, Latawiec A, Misselbrook T, Phalan B, Simmons BI, Takahashi T, Vause J (2018) The environmental costs and benefits of high-yield farming. Nat Sustain 1(9):477–485. https://doi.org/10.1038/s41893-018-0138-5
Bardhan K, Patel DP (2016) Urgency to understand nitrogen metabolism in organic agriculture. Adv Crop Sci Technol. https://doi.org/10.4172/2329-8863.1000236
Batish DR, Singh HP, Kaur S, Kohli RK, Yadav SS (2008) Caffeic acid affects early growth, and morphogenetic response of hypocotyl cuttings of mung bean (Phaseolus aureus). J Plant Physiol 165(3):297–305. https://doi.org/10.1016/j.jplph.2007.05.003
Battye W, Aneja VP, Schlesinger WH (2017) Is nitrogen the next carbon? Earths Future 5(9):894–904. https://doi.org/10.1002/2017ef000592
Bhogal A, Williams J, Nicholson F, Chadwick D, Chambers K, Chambers B (2016) Mineralization of organic nitrogen from farm manure applications. Soil Use Manag 32(S1):32–43. https://doi.org/10.1111/sum.12263
Blanco-Canqui H, Shaver TM, Lindquist JL, Shapiro CA, Elmore RW, Francis CA, Hergert GW (2015) Cover crops and ecosystem services: insights from studies in temperate soils. Agron J 107(6):2449–2474. https://doi.org/10.2134/agronj15.0086
Bowles TM, Atallah SS, Campbell EE, Gaudin ACM, Wieder WR, Grandy AS (2018) Addressing agricultural nitrogen losses in a changing climate. Nat Sustain 1(8):399–408. https://doi.org/10.1038/s41893-018-0106-0
Burgt GJ, Rietema C, Bus M (2018) Planty organic 5 year: evaluation of soil fertility, nitrogen dynamics and production
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2013.0122
Cambui CA, Svennerstam H, Gruffman L, Nordin A, Ganeteg U, Näsholm T (2011) Patterns of plant biomass partitioning depend on nitrogen source. PLoS ONE 6(4):e19211. https://doi.org/10.1371/journal.pone.0019211
Cameron KC, Di HJ, Moir JL (2013) Nitrogen losses from the soil/plant system: a review. Ann Appl Biol 162(2):145–173. https://doi.org/10.1111/aab.12014
Campiglia E, Mancinelli R, Radicetti E, Marinari S (2011) Legume cover crops and mulches: effects on nitrate leaching and nitrogen input in a pepper crop (Capsicum annuum L.). Nutr Cycl Agroecosyst 89(3):399–412. https://doi.org/10.1007/s10705-010-9404-2
Carr PM, Cavigelli MA, Darby H, Delate K, Eberly JO, Fryer HK, Gramig GG, Heckman JR, Mallory EB, Reeve JR, Silva EM, Suchoff DH, Woodley AL (2020) Green and animal manure use in organic field crop systems. Agron J 112(2):648–674. https://doi.org/10.1002/agj2.20082
Carter MS, Sorensen P, Petersen SO, Ma XZ, Ambus P (2014) Effects of green manure storage and incorporation methods on nitrogen release and N2O emissions after soil application. Biol Fertil Soils 50(8):1233–1246. https://doi.org/10.1007/s00374-014-0936-5
Cassman KG, Dobermann A, Walters DT (2002) Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 31(2):132–140. https://doi.org/10.1579/0044-7447-31.2.132
Caulfield M, Groot JCJ, Fonte SJ, Sherwood S, Oyarzun P, Borja RM, Dumble S, Tittonell P (2020) Live barriers and associated organic amendments mitigate land degradation and improve crop productivity in hillside agricultural systems of the Ecuadorian Andes. Land Degrad Dev 31(13):1650–1661. https://doi.org/10.1002/ldr.3558
Celestina C, Hunt JR, Sale PWG, Franks AE (2019) Attribution of crop yield responses to application of organic amendments: a critical review. Soil Tillage Res 186:135–145. https://doi.org/10.1016/j.still.2018.10.002
Chen HH, Li XC, Hu F, Shi W (2013) Soil nitrous oxide emissions following crop residue addition: a meta-analysis. Glob Change Biol 19(10):2956–2964. https://doi.org/10.1111/gcb.12274
Chen BQ, Liu EK, Tian QZ, Yan CR, Zhang YQ (2014) Soil nitrogen dynamics and crop residues: a review. Agron Sustain Dev 34(2):429–442. https://doi.org/10.1007/s13593-014-0207-8
Chen HH, Dai ZM, Veach AM, Zheng JQ, Xu JM, Schadt CW (2020) Global meta-analyses show that conservation tillage practices promote soil fungal and bacterial biomass. Agric Ecosyst Environ. https://doi.org/10.1016/j.agee.2020.106841
Chomel M, Guittonny-Larcheveque M, Fernandez C, Gallet C, DesRochers A, Pare D, Jackson BG, Baldy V (2016) Plant secondary metabolites: a key driver of litter decomposition and soil nutrient cycling. J Ecol 104(6):1527–1541. https://doi.org/10.1111/1365-2745.12644
Clarholm M (1985) Interactions of bacteria, protozoa and plants leading to mineralization of soil-nitrogen. Soil Biol Biochem 17(2):181–187. https://doi.org/10.1016/0038-0717(85)90113-0
Congreves KA, Eerd LL (2015) Nitrogen cycling and management in intensive horticultural systems. Nutr Cycl Agroecosyst 102(3):299–318. https://doi.org/10.1007/s10705-015-9704-7
Congreves KA, Otchere O, Ferland D, Farzadfar S, Williams S, Arcand MM (2021) Nitrogen use efficiency definitions of today and tomorrow. Front Plant Sci. https://doi.org/10.3389/fpls.2021.637108
Cooper H, Sjögersten S, Lark R, Mooney S (2021) To till or not to till in a temperate ecosystem? Implications for climate change mitigation. Environ Res Lett 16(5):054022. https://doi.org/10.1088/1748-9326/abe74e
Coppens F, Garnier P, De Gryze S, Merckx R, Recous S (2006) Soil moisture, carbon and nitrogen dynamics following incorporation and surface application of labelled crop residues in soil columns. Eur J Soil Sci 57(6):894–905. https://doi.org/10.1111/j.1365-2389.2006.00783.x
Coppens F, Garnier P, Findeling A, Merckx R, Recous S (2007) Decomposition of mulched versus incorporated crop residues: modelling with PASTIS clarifies interactions between residue quality and location. Soil Biol Biochem 39(9):2339–2350. https://doi.org/10.1016/j.soilbio.2007.04.005
Cordero RL, Torchelsen FP, Overbeck GE, Anand M (2016) Analyzing the landscape characteristics promoting the establishment and spread of gorse (Ulex europaeus) along roadsides. Ecosphere. https://doi.org/10.1002/ecs2.1201
Crews TE, Peoples MB (2004) Legume versus fertilizer sources of nitrogen: ecological tradeoffs and human needs. Agric Ecosyst Environ 102(3):279–297. https://doi.org/10.1016/j.agee.2003.09.018
Crews TE, Peoples MB (2005) Can the synchrony of nitrogen supply and crop demand be improved in legume and fertilizer-based agroecosystems? A review. Nutr Cycl Agroecosyst 72(2):101–120. https://doi.org/10.1007/s10705-004-6480-1
Crippa M, Solazzo E, Guizzardi D, Monforti-Ferrario F, Tubiello FN, Leip A (2021) Food systems are responsible for a third of global anthropogenic GHG emissions. Nat Food 2(3):198–209. https://doi.org/10.1038/s43016-021-00225-9
De Neve S, Hofman G (2002) Quantifying soil water effects on nitrogen mineralization from soil organic matter and from fresh crop residues. Biol Fertil Soils 35(5):379–386. https://doi.org/10.1007/s00374-002-0483-3
De Notaris C, Mortensen EO, Sorensen P, Olesen JE, Rasmussen J (2021) Cover crop mixtures including legumes can self-regulate to optimize N-2 fixation while reducing nitrate leaching. Agric Ecosyst Environ. https://doi.org/10.1016/j.agee.2020.107287
de Ruijter FJ, Huijsmans JFM, Rutgers B (2010) Ammonia volatilization from crop residues and frozen green manure crops. Atmos Environ 44(28):3362–3368. https://doi.org/10.1016/j.atmosenv.2010.06.019
Debaeke P, Rouet P, Justes E (2006) Relationship between the normalized SPAD index and the nitrogen nutrition index: application to durum wheat. J Plant Nutr 29(1):75–92. https://doi.org/10.1080/01904160500416471
Defra (Department for Environment, Food and Rural Affairs, London, UK) (2010) Fertiliser manual RB209. https://www.rothamsted.ac.uk/sites/default/files/rb209-fertiliser-manual-110412.pdf
Defra (Department for Environment, Food and Rural Affairs, London, UK) (2019) Farming statistics final crop areas, yields, livestock populations and agricultural workforce at June 2018—United Kingdom. Accessed at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/869062/structure-jun2018final-uk-28feb20.pdf. Accessed on 20 Aug 2022
Defra (Department for Environment, Food and Rural Affairs, London, UK) (2022) Invasive non-native (alien) plant species: rules in England and Wales. Accessed at https://www.gov.uk/guidance/invasive-non-native-alien-plant-species-rules-in-england-and-wales#restrictions. Acceessed on 20 Aug 2022
Di Lonardo DP, De Boer W, Gunnewiek P, Hannula SE, Van der Wal A (2017) Priming of soil organic matter: chemical structure of added compounds is more important than the energy content. Soil Biol Biochem 108:41–54. https://doi.org/10.1016/j.soilbio.2017.01.017
Di HJ, Cameron KC (2002) Nitrate leaching in temperate agroecosystems: sources, factors and mitigating strategies. Nutr Cycl Agroecosyst 64(3):237–256. https://doi.org/10.1023/a:1021471531188
Diederich KM, Ruark MD, Krishnan K, Arriaga FJ, Silva EM (2019) Increasing labile soil carbon and nitrogen fractions require a change in system, rather than practice. Soil Sci Soc Am J 83(6):1733–1745. https://doi.org/10.2136/sssaj2018.11.0458
Dodd IC, Whalley WR, Ober ES, Parry MAJ (2011) Genetic and management approaches to boost UK wheat yields by ameliorating water deficits. J Exp Bot 62(15):5241–5248. https://doi.org/10.1093/jxb/err242
Farzadfar S, Knight JD, Congreves KA (2021) Soil organic nitrogen: an overlooked but potentially significant contribution to crop nutrition. Plant Soil 462(1–2):7–23. https://doi.org/10.1007/s11104-021-04860-w
Finlay KW, Wilkinson GN (1963) The analysis of adaptation in a plant-breeding programme. Crop Pasture Sci 14(6):742–754. https://doi.org/10.1071/AR9630742
Finney DM, Eckert SE, Kaye JP (2015) Drivers of nitrogen dynamics in ecologically based agriculture revealed by long-term, high-frequency field measurements. Ecol Appl 25(8):2210–2227. https://doi.org/10.1890/14-1357.1.sm
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35(6):837–843. https://doi.org/10.1016/s0038-0717(03)00123-8
Fowler D, Coyle M, Skiba U, Sutton MA, Cape JN, Reis S, Sheppard LJ, Jenkins A, Grizzetti B, Galloway JN, Vitousek P, Leach A, Bouwman AF, Butterbach-Bahl K, Dentener F, Stevenson D, Amann M, Voss M (2013) The global nitrogen cycle in the twenty-first century. Philos Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2013.0164
Fox RH, Myers RJK, Vallis I (1990) The nitrogen mineralization rate of legume residues in soil as influenced by their polyphenol, lignin, and nitrogen contents. Plant Soil 129(2):251–259. https://doi.org/10.1007/BF00032420
Franklin O, Cambui CA, Gruffman L, Palmroth S, Oren R, Nasholm T (2017) The carbon bonus of organic nitrogen enhances nitrogen use efficiency of plants. Plant Cell Environ 40(1):25–35. https://doi.org/10.1111/pce.12772
Friedel JK, Ardakani MR (2021) Soil nutrient dynamics and plant-induced nutrient mobilisation in organic and low-input farming systems: conceptual framework and relevance. Biol Agric Hortic 37(1):1–24. https://doi.org/10.1080/01448765.2020.1855247
Gunnarsson S, Marstorp H (2002) Carbohydrate composition of plant materials determines N mineralisation. Nutr Cycl Agroecosyst 62(2):175–183. https://doi.org/10.1023/a:1015512106336
Handayanto E, Giller KE, Cadisch G (1997) Regulating N release from legume tree prunings by mixing residues of different quality. Soil Biol Biochem 29(9–10):1417–1426. https://doi.org/10.1016/s0038-0717(97)00047-3
Hansen S, Froseth RB, Stenberg M, Stalenga J, Olesen JE, Krauss M, Radzikowski P, Doltra J, Nadeem S, Torp T, Pappa V, Watson CA (2019) Reviews and syntheses: review of causes and sources of N2O emissions and NO3 leaching from organic arable crop rotations. Biogeosciences 16(14):2795–2819. https://doi.org/10.5194/bg-16-2795-2019
Hartz T (2007) Efficient nitrogen management for cool-season vegetables. University of California, Department of plant Science. UC Davis
Hayek MN, Harwatt H, Ripple WJ, Mueller ND (2021) The carbon opportunity cost of animal-sourced food production on land. Nat Sustain 4(1):21–24. https://doi.org/10.1038/s41893-020-00603-4
Hill PW, Quilliam RS, DeLuca TH, Farrar J, Farrell M, Roberts P, Newsham KK, Hopkins DW, Bardgett RD, Jones DL (2011) Acquisition and assimilation of nitrogen as peptide-bound and D-enantiomers of amino acids by wheat. PLoS ONE. https://doi.org/10.1371/journal.pone.0019220
Hirel B, Tetu T, Lea PJ, Dubois F (2011) Improving nitrogen use efficiency in crops for sustainable agriculture. Sustainability 3(9):1452–1485. https://doi.org/10.3390/su3091452
Hodge A, Robinson D, Griffiths BS, Fitter AH (1999) Why plants bother: root proliferation results in increased nitrogen capture from an organic patch when two grasses compete. Plant Cell Environ 22(7):811–820. https://doi.org/10.1046/j.1365-3040.1999.00454.x
Houlton BZ, Almaraz M, Aneja V, Austin AT, Bai E, Cassman KG, Compton JE, Davidson EA, Erisman JW, Galloway JN, Gu BJ, Yao GL, Martinelli LA, Scow K, Schlesinger WH, Tomich TP, Wang C, Zhang X (2019) A world of cobenefits: solving the global nitrogen challenge. Earths Future 7(8):865–872. https://doi.org/10.1029/2019ef001222
Houston Durrant T, de Rigo D, Caudullo G (2016) Alnus glutinosa in Europe: distribution, habitat, usage and threats. European Atlas of Forest Tree Species. Publ. Off. EU, Luxembourg, pp e01f3c0
Hoyle FC, Murphy DV (2011) Influence of organic residues and soil incorporation on temporal measures of microbial biomass and plant available nitrogen. Plant Soil 347(1–2):53–64. https://doi.org/10.1007/s11104-011-0922-8
IUSS Working Group WRB (2015) World reference base for soil resources 2014, Update 2015. In: International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. FAO, Rome. Accessed at https://www.fao.org/3/i3794en/I3794en.pdf. Accessed on 29 Mar 2022
Jackson LE, Burger M, Cavagnaro TR (2008) Roots nitrogen transformations, and ecosystem services. Annu Rev Plant Biol 59:341–363. https://doi.org/10.1146/annurev.arplant.59.032607.092932
Kaewpradit W, Toomsan B, Vityakon P, Limpinuntana V, Saenjan P, Jogloy S, Patanothai A, Cadisch G (2008) Regulating mineral N release and greenhouse gas emissions by mixing groundnut residues and rice straw under field conditions. Eur J Soil Sci 59(4):640–652. https://doi.org/10.1111/j.1365-2389.2008.01021.x
Kibblewhite MG, Ritz K, Swift MJ (2008) Soil health in agricultural systems. Philos Trans R Soc B Biol Sci 363(1492):685–701. https://doi.org/10.1098/rstb.2007.2178
Kubota H, Iqbal M, Quideau S, Dyck M, Spaner D (2018) Agronomic and physiological aspects of nitrogen use efficiency in conventional and organic cereal-based production systems. Renew Agric Food Syst 33(5):443–466. https://doi.org/10.1017/s1742170517000163
Kumar K, Goh K (1999) Crop residues and management practices: effects on soil quality, soil nitrogen dynamics, crop yield, and nitrogen recovery. Adv Agron 68:197–319. https://doi.org/10.1016/S0065-2113(08)60846-9
Lal R (2004) Soil carbon sequestration to mitigate climate change. Geoderma 123(1–2):1–22. https://doi.org/10.1016/j.geoderma.2004.01.032
Lamb A, Green R, Bateman I, Broadmeadow M, Bruce T, Burney J, Carey P, Chadwick D, Crane E, Field R, Goulding K, Griffiths H, Hastings A, Kasoar T, Kindred D, Phalan B, Pickett J, Smith P, Wall E, Ermgassen E, Balmford A (2016) The potential for land sparing to offset greenhouse gas emissions from agriculture. Nat Clim Chang 6(5):488–492. https://doi.org/10.1038/nclimate2910
Larsson L, Ferm M, Kasimir-Klemedtsson A, Klemedtsson L (1998) Ammonia and nitrous oxide emissions from grass and alfalfa mulches. Nutr Cycl Agroecosyst 51(1):41–46. https://doi.org/10.1023/a:1009799126377
Lehtinen T, Schlatter N, Baumgarten A, Bechini L, Kruger J, Grignani C, Zavattaro L, Costamagna C (2014) Effect of crop residue incorporation on soil organic carbon and greenhouse gas emissions in European agricultural soils. Soil Use Manag 30(4):524–538. https://doi.org/10.1111/sum.12151
Lemtiri A, Degrune F, Barbieux S, Hiel MP, Chélin M, Parvin N, Vandenbol M, Francis F, Colinet G (2016) Crop residue management in arable cropping systems under temperate climate, part 1: soil biological and chemical (phosphorus and nitrogen) properties: a review. Biotechnol Agron Soc Environ. https://doi.org/10.25518/1780-4507.13015
Li FC, Sorensen P, Li XX, Olesen JE (2020) Carbon and nitrogen mineralization differ between incorporated shoots and roots of legume versus non-legume based cover crops. Plant Soil 446(1–2):243–257. https://doi.org/10.1007/s11104-019-04358-6
Lin HC, Huber JA, Gerl G, Hulsbergen KJ (2016) Nitrogen balances and nitrogen-use efficiency of different organic and conventional farming systems. Nutr Cycl Agroecosyst 105(1):1–23. https://doi.org/10.1007/s10705-016-9770-5
Loboguerrero AM, Campbell BM, Cooper PJM, Hansen JW, Rosenstock T, Wollenberg E (2019) Food and earth systems: priorities for climate change adaptation and mitigation for agriculture and food systems. Sustainability. https://doi.org/10.3390/su11051372
Lonhienne TGA, Trusov Y, Young A, Rentsch D, Nasholm T, Schmidt S, Paungfoo-Lonhienne C (2014) Effects of externally supplied protein on root morphology and biomass allocation in Arabidopsis. Sci Rep. https://doi.org/10.1038/srep05055
Lupwayi NZ, Soon YK (2015) Carbon and nitrogen release from legume crop residues for three subsequent crops. Soil Sci Soc Am J 79(6):1650–1659. https://doi.org/10.2136/sssaj2015.05.0198
Ma QX, Wu LH, Wang J, Ma JZ, Zheng NG, Hill PW, Chadwick DR, Jones DL (2018) Fertilizer regime changes the competitive uptake of organic nitrogen by wheat and soil microorganisms: an in-situ uptake test using C-13, N-15 labelling, and C-13-PLFA analysis. Soil Biol Biochem 125:319–327. https://doi.org/10.1016/j.soilbio.2018.08.009
Marshall MR, Ballard CE, Frogbrook ZL, Solloway I, McIntyre N, Reynolds B, Wheater HS (2014) The impact of rural land management changes on soil hydraulic properties and runoff processes: results from experimental plots in upland UK. Hydrol Process 28(4):2617–2629. https://doi.org/10.1002/hyp.9826
McClelland SC, Paustian K, Schipanski ME (2021) Management of cover crops in temperate climates influences soil organic carbon stocks: a meta-analysis. Ecol Appl. https://doi.org/10.1002/eap.2278
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide Biol Chem 5(1):62–71. https://doi.org/10.1006/niox.2000.0319
Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76(2):151–174. https://doi.org/10.1890/0012-9615(2006)076[0151:atmold]2.0.co;2
Moreau D, Bardgett RD, Finlay RD, Jones DL, Philippot L (2019) A plant perspective on nitrogen cycling in the rhizosphere. Funct Ecol 33(4):540–552. https://doi.org/10.1111/1365-2435.13303
Mosier A, Syers JK, Freney JR (2013) Agriculture and the nitrogen cycle: assessing the impacts of fertilizer use on food production and the environment, vol 65, Island Press
Mὃller K (2018) Soil fertility status and nutrient input-output flows of specialised organic cropping systems: a review. Nutr Cycl Agroecosyst 112(2):147–164. https://doi.org/10.1007/s10705-018-9946-2
Mὃller K, Stinner W (2009) Effects of different manuring systems with and without biogas digestion on soil mineral nitrogen content and on gaseous nitrogen losses (ammonia, nitrous oxides). Eur J Agron 30(1):1–16. https://doi.org/10.1016/j.eja.2008.06.003
Neeteson JJ, Carton OT (2001) The environmental impact of nitrogen in field vegetable production. In: Proceedings of the international conference on environmental problems associated with nitrogen fertilisation of field grown vegetable crops, vol 563, pp 21–28. https://doi.org/10.17660/ActaHortic.2001.563.1
Nett L, Fuss R, Flessa H, Fink M (2015) Emissions of nitrous oxide and ammonia from a sandy soil following surface application and incorporation of cauliflower leaf residues. J Agric Sci 153(8):1341–1352. https://doi.org/10.1017/s0021859615000027
Nett L, Sradnick A, Fuss R, Flessa H, Fink M (2016) Emissions of nitrous oxide and ammonia after cauliflower harvest are influenced by soil type and crop residue management. Nutr Cycl Agroecosyst 106(2):217–231. https://doi.org/10.1007/s10705-016-9801-2
Nicolardot B, Recous S, Mary B (2001) Simulation of C and N mineralisation during crop residue decomposition: a simple dynamic model based on the C:N ratio of the residues. Plant Soil 228(1):83–103. https://doi.org/10.1023/a:1004813801728
NNSS (2011) Giant rhubarbs—species description. GB non-native species secretariat. Accessed at https://www.nonnativespecies.org/assets/Uploads/ID_Gunnera_species_Giant_rhubarb-v2.pdf. Accessed on 29 Mar 2022
Norton J, Ouyang Y (2019) Controls and adaptive management of nitrification in agricultural soils. Front Microbiol. https://doi.org/10.3389/fmicb.2019.01931
Palm CA (1995) Contribution of agroforestry trees to nutrient-requirements of intercropped plants. Agrofor Syst 30(1–2):105–124. https://doi.org/10.1007/bf00708916
Palm CA, Gachengo CN, Delve RJ, Cadisch G, Giller KE (2001) Organic inputs for soil fertility management in tropical agroecosystems: application of an organic resource database. Agric Ecosyst Environ 83(1–2):27–42. https://doi.org/10.1016/s0167-8809(00)00267-x
Palozzi JE, Lindo Z (2018) Are leaf litter and microbes team players? Interpreting home-field advantage decomposition dynamics. Soil Biol Biochem 124:189–198. https://doi.org/10.1016/j.soilbio.2018.06.018
Pan BB, Lam SK, Mosier A, Luo YQ, Chen DL (2016) Ammonia volatilization from synthetic fertilizers and its mitigation strategies: a global synthesis. Agric Ecosyst Environ 232:283–289. https://doi.org/10.1016/j.agee.2016.08.019
Pardo-Muras M, Puig CG, Lopez-Nogueira A, Cavaleiro C, Pedrol N (2018) On the bioherbicide potential of ulex europaeus and Cytisus scoparius: profiles of volatile organic compounds and their phytotoxic effects. PLoS ONE. https://doi.org/10.1371/journal.pone.0205997
Pardo-Muras M, Puig CG, Souza-Alonso P, Pedrol N (2020) The phytotoxic potential of the flowering foliage of gorse (Ulex europaeus) and scotch broom (Cytisus scoparius), as pre-emergent weed control in maize in a glasshouse pot experiment. Plants-Basel. https://doi.org/10.3390/plants9020203
Paungfoo-Lonhienne C, Visser J, Lonhienne TGA, Schmidt S (2012) Past, present and future of organic nutrients [review]. Plant Soil 359(1–2):1–18. https://doi.org/10.1007/s11104-012-1357-6
Pilling D, Bélanger J, Hoffmann I (2020) Declining biodiversity for food and agriculture needs urgent global action. Nat Food 1(3):144–147
Powlson DS, Gregory PJ, Whalley WR, Quinton JN, Hopkins DW, Whitmore AP, Hirsch PR, Goulding KWT (2011) Soil management in relation to sustainable agriculture and ecosystem services. Food Policy 36:S72–S87. https://doi.org/10.1016/j.foodpol.2010.11.025
Pullens JWM, Sorensen P, Melander B, Olesen JE (2021) Legacy effects of soil fertility management on cereal dry matter and nitrogen grain yield of organic arable cropping systems. Eur J Agron. https://doi.org/10.1016/j.eja.2020.126169
Rahn C (2012) “Nitrogen Requirement for Leeks” HDC factsheet 32/12 Project FV 350 Agriculture and Horticulture Development Board Warwick HRI, University of Warwick, Wellesbourne, Warwick, CV35 9EF. Accessed at https://projectblue.blob.core.windows.net/media/Default/Imported%20Publication%20Docs/32_12%20Nitrogen%20requirements%20for%20leeks.pdf. Accessed on 17 Sep 2020
Rees RM, Augustin J, Alberti G, Ball BC, Boeckx P, Cantarel A, Castaldi S, Chirinda N, Chojnicki B, Giebels M, Gordon H, Grosz B, Horvath L, Juszczak R, Klemedtsson AK, Klemedtsson L, Medinets S, Machon A, Mapanda F, Nyamangara J, Olesen JE, Reay DS, Sanchez L, Cobena AS, Smith KA, Sowerby A, Sommer M, Soussana JF, Stenberg M, Topp CFE, van Cleemput O, Vallejo A, Watson CA, Wuta M (2013) Nitrous oxide emissions from European agriculture—an analysis of variability and drivers of emissions from field experiments. Biogeosciences 10(4):2671–2682. https://doi.org/10.5194/bg-10-2671-2013
Rees RM, Maire J, Florence A, Cowan N, Skiba U, Vanderweerden T, Ju XT (2020) Mitigating nitrous oxide emissions from agricultural soils by precision management. Front Agric Sci Eng 7(1):75–80
Rigby H, Smith SR (2014) The nitrogen fertiliser value and other agronomic benefits of industrial biowastes. Nutr Cycl Agroecosyst 98(2):137–154. https://doi.org/10.1007/s10705-014-9602-4
Robertson GP, Groffman PM (2007) Nitrogen transformations. In Groffman PM (ed) Soil microbiology, ecology, and biochemistry, vol 3, pp 341–364. https://doi.org/10.1016/B978-0-08-047514-1.50017-2
Robinson D (1994) The responses of plants to nonuniform supplies of nutrients. New Phytol 127(4):635–674. https://doi.org/10.1111/j.1469-8137.1994.tb02969.x
Rockstrom J, Steffen W, Noone K, Persson A, Chapin FS, Lambin E, Lenton TM, Scheffer M, Folke C, Schellnhuber HJ, Nykvist B, de Wit AC, Hughes T, van der Leeuw S, Rodhe H, Sorlin S, Snyder PK, Costanza R, Svedin U, Falkenmark M, Karlberg L, Corell RW, Fabry VJ, Hansen J, Walker B, Liverman D, Richardson K, Crutzen P, Foley J (2009) Planetary boundaries: exploring the safe operating space for humanity. Ecol Soc 14:2
Roger-Estrade J, Anger C, Bertrand M, Richard G (2010) Tillage and soil ecology: partners for sustainable agriculture [review]. Soil Tillage Res 111(1):33–40. https://doi.org/10.1016/j.still.2010.08.010
Rothe M, Darnaudery M, Thuries L (2019) Organic fertilizers, green manures and mixtures of the two revealed their potential as substitutes for inorganic fertilizers used in pineapple cropping. Scientia Horticulturae. https://doi.org/10.1016/j.scienta.2019.108691
Sánchez-Rodríguez AR, Carswell AM, Shaw R, Hunt J, Saunders K, Cotton J, Chadwick DR (2018) Advanced processing of food waste based digestate for mitigating nitrogen losses in a winter wheat crop. Front Sustain Food Syst 2:35. https://doi.org/10.3389/fsufs.2018.00035
Schmidt S, Raven JA, Paungfoo-Lonhienne C (2013) The mixotrophic nature of photosynthetic plants. Funct Plant Biol 40(5):425–438. https://doi.org/10.1071/fp13061
Sebilo M, Mayer B, Nicolardot B, Pinay G, Mariotti A (2013) Long-term fate of nitrate fertilizer in agricultural soils. Proc Natl Acad Sci USA 110(45):18185–18189. https://doi.org/10.1073/pnas.1305372110
Shan J, Yan X (2013) Effects of crop residue returning on nitrous oxide emissions in agricultural soils. Atmos Environ 71:170–175. https://doi.org/10.1016/j.atmosenv.2013.02.009
Sileshi GW, Akinnifesi FK, Mafongoya PL, Kuntashula E, Ajayi OC (2020) Potential of gliricidia-based agroforestry systems for resource-limited agroecosystems. In Dagar JC, Gupta SR, Teketay D (eds) Agroforestry for degraded landscapes: recent advances and emerging challenges, vol 1, Springer, Singapore, pp 255–282. https://doi.org/10.1007/978-981-15-4136-0_9
Smil V (2011) Nitrogen cycle and world food production. World Agric 2(1):9–13
Smith LG, Jones PJ, Kirk GJD, Pearce BD, Williams AG (2018) Modelling the production impacts of a widespread conversion to organic agriculture in England and Wales. Land Use Policy 76:391–404. https://doi.org/10.1016/j.landusepol.2018.02.035
Sorensen JN, Thorup-Kristensen K (2011) Plant-based fertilizers for organic vegetable production. J Plant Nutr Soil Sci 174(2):321–332. https://doi.org/10.1002/jpln.200900321
Swain T, Hillis W (1959) The phenolic constituents of Prunus domestica, I: the quantitative analysis of phenolic constituents. J Sci Food Agric 10(1):63–68. https://doi.org/10.1002/jsfa.2740100110
Tei F, De Neve S, de Haan J, Kristensen HL (2020) Nitrogen management of vegetable crops. Agric Water Manag. https://doi.org/10.1016/j.agwat.2020.106316
Trinsoutrot I, Recous S, Bentz B, Lineres M, Cheneby D, Nicolardot B (2000) Biochemical quality of crop residues and carbon and nitrogen mineralization kinetics under nonlimiting nitrogen conditions. Soil Sci Soc Am J 64(3):918–926. https://doi.org/10.2136/sssaj2000.643918x
Truong THH, Marschner P (2018) Respiration, available N and microbial biomass N in soil amended with mixes of organic materials differing in C/N ratio and decomposition stage. Geoderma 319:167–174. https://doi.org/10.1016/j.geoderma.2018.01.012
Udvardi M, Below FE, Castellano MJ, Eagle AJ, Giller KE, Ladha JK, Liu XJ, Maaz TM, Nova-Franco B, Raghuram N, Robertson GP, Roy S, Saha M, Schmidt S, Tegeder M, York LM, Peters JW (2021) A research road map for responsible use of agricultural nitrogen. Front Sustain Food Syst. https://doi.org/10.3389/fsufs.2021.660155
van Kessel C, Clough T, van Groenigen JW (2009) Dissolved organic nitrogen: an overlooked pathway of nitrogen loss from agricultural systems? J Environ Qual 38(2):393–401. https://doi.org/10.2134/jeq2008.0277
Vessey JK, Pawlowski K, Bergman B (2005) Root-based N-2-fixing symbioses: legumes, actinorhizal plants, Parasponia sp. and cycads [review]. Plant Soil 274(1–2):51–78. https://doi.org/10.1007/s11104-005-5881-5
Whalen JK (2014) Managing soil biota-mediated decomposition and nutrient mineralization in sustainable agroecosystems. Adv Agric. https://doi.org/10.1155/2014/384604
Willett W, Rockstrom J, Loken B (2019) Food in the anthropocene: the EAT-lancet commission on healthy diets from sustainable food systems. Lancet 393(10171):530–530. https://doi.org/10.1016/S0140-6736(18)31788-4
Yan M, Pan GX, Lavallee JM, Conant RT (2020) Rethinking sources of nitrogen to cereal crops. Glob Change Biol 26(1):191–199. https://doi.org/10.1111/gcb.14908
Zhang JE, Lu CQ, Feng HL, Hennessy D, Guan Y, Wright MM (2021) Extreme climate increased crop nitrogen surplus in the United States. Agric Forest Meteorol. https://doi.org/10.1016/j.agrformet.2021.108632
Zhu B, Gutknecht JLM, Herman DJ, Keck DC, Firestone MK, Cheng WX (2014) Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biol Biochem 76:183–192. https://doi.org/10.1016/j.soilbio.2014.04.033
Acknowledgements
CRW was financially supported through a Knowledge Economy Skills Scholarships (KESS 2), with the Centre for Alternative Technology (Machynlleth, Powys, UK) acting as the partner organisation. KESS 2 is a pan-Wales higher level skills initiative led by Bangor University on behalf of the HE sector in Wales. It is part funded by the Welsh Government’s European Social Fund (ESF) convergence programme for West Wales and the Valleys.
Author information
Authors and Affiliations
Contributions
CRW conceived the techniques researched using Perennial Mobile Green Manures. Experiments were devised by CRW, PWH and DRC. Experimental work, data processing, and writing were undertaken by CRW, with advice and edits by PWH and DRC.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ward, C.R., Chadwick, D.R. & Hill, P.W. Potential to improve nitrogen use efficiency (NUE) by use of perennial mobile green manures. Nutr Cycl Agroecosyst 125, 43–62 (2023). https://doi.org/10.1007/s10705-022-10253-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-022-10253-x