Abstract
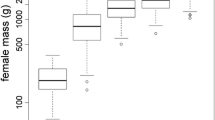
Amphibian reproductive modes are diverse and are characterised by complex adaptations, including vast variability in life history traits and different parental investment strategies. For instance, viviparity is rare in urodeles despite the potential ecological advantages gained in such populations by having semi-independency from water. The fire salamander, Salamandra salamandra, shows remarkable intraspecific variation in reproductive modes, with two strategies co-occurring: a common reproductive mode, larviparity (parturition of aquatic larvae), and a phylogenetically derived reproductive mode, pueriparity (parturition of terrestrial juveniles). Pueriparous populations of S. salamandra have at least two independent origins, the first originating from its northern distribution in the Iberian Peninsula, and the second at two insular populations on the northwestern Iberian coast. Here, we analyse the patterns of variability of some life-history traits in larviparous and pueriparous populations of S. salamandra, including pueriparous populations from the two independent origins, to understand how these traits relate to the evolutionary transitions in reproductive modes in S. salamandra. Our study shows differences in female body size and clutch and brood size between larviparous and pueriparous fire salamanders. We did not find differences in female investment between reproductive modes, and thus, the evolution to pueriparity in S. salamandra is likely characterised by the re-allocation of eggs to matrotrophy. Our study also confirms pueriparity and larviparity as the characteristic reproductive modes for insular and coastal/mainland S. s. gallaica populations, respectively, revealing the potential presence of pueriparity in one coastal population. This comparative analysis sheds light on the maternal factors that might have driven, or are related to, the evolution of pueriparity in this unique biological system and sets up the basis for testing different hypotheses that include climatic, ecological, physiological, and genetic factors as drivers of this evolutionary transition.



Similar content being viewed by others
References
Alcobendas M, Castanet J (2000) Bone growth plasticity among populations of Salamandra salamandra: interactions between internal and external factors. Herpetologica 56:14–26
Alcobendas M, Dopazo H, Alberch P (1996) Geographic variation in allozymes of populations of Salamandra salamandra (Amphibia: Urodela) exhibiting distinct reproductive modes. J Evol Biol 9:83–102
Alcobendas M, Buckley D, Tejedo M (2004) Variability in survival, growth and metamorphosis in the larval fire salamander (Salamandra salamandra): effects of larval birth size, sibship and environment. Herpetologica 60:232–245
AmphibiaWeb (2014) Information on amphibian biology and conservation. Berkeley, CA. http://amphibiaweb.org. Accessed 22 Jan 2014
Beukema W, de Pous P, Donaire D et al (2010) Biogeography and contemporary climatic differentiation among Moroccan Salamandra algira. Biol J Linn Soc 101:626–641
Blackburn DG (2000) Reptilian viviparity: past research, future directions, and appropriate models. Comp Biochem Phys A Mol Int Phys 127:391–409
Blackburn DG (2014) Evolution of vertebrate viviparity and specializations for fetal nutrition: a quantitative and qualitative analysis. J Morphol. doi:10.1002/jmor.20272
Bleu J, Heulin B, Haussy C, Meylan S, Massot M (2012) Experimental evidence of early costs o reproduction in conspecific viviparous and oviparous lizards. J Evol Biol 25:1264–1274
Buckley D (2003) La evolución del viviparismo en Salamandra salamandra (Linnaeus, 1758). PhD Dissertation. Universidad Autónoma de Madrid, Spain
Buckley D (2012) Evolution of viviparity in salamanders (Amphibia, Caudata). In: eLS. Wiley, Chichester. doi:10.1002/9780470015902.a0022851
Buckley D, Alcobendas M, García-París M, Wake MH (2007) Heterochrony, cannibalism, and the evolution of viviparity in Salamandra salamandra. Evol Dev 9:105–115
Caley MJ, Schwarzkopf L, Shine R (2001) Does total reproductive effort evolve independently of offspring size? Evolution 55:1245–1248
Clutton-Brock TH (2001) The evolution of parental care. Princeton University Press, Princeton, NJ
Cordero Rivera A, Velo-Antón G, Galán P (2007) Ecology of amphibians in small coastal Holocene islands: local adaptations and the effect of exotic tree plantations. Munibe 25:94–103
Cunningham EJ (2002) Aves (birds). In: eLS. Wiley, Chichester. doi:10.1038/npg.els.0001548
Dias JMA, Boski T, Rodrigues A et al (2000) Coast line evolution in Portugal since the last glacial maximum until present: a synthesis. Mar Geol 170:177–186
Dopazo HJ, Alberch P (1994) Preliminary results on optional viviparity and intrauterine siblicide in Salamandra salamandra populations from northern Spain. Mertensiella 4:125–138
Dopazo HJ, Korenblum M (2000) Viviparity in Salamandra salamandra (Amphibia: Salamandridae): adaptation or exaptation? Herpetologica 56:144–152
Galán P (2007) Viviparismo y distribución de Salamandra salamandra bernardezi en el norte de Galicia. Bol Asoc Esp Herp 18:44–48
Galán P, Velo-Antón G, Cordero Rivera A (2011) Puesta de huevos infecundos en Salamandra salamandra. Bol Asoc Esp Herp 22:1–3
García-París M, Alcobendas M, Buckley D, Wake DB (2003) Dispersal of viviparity across contact zones in Iberian populations of fire salamanders (Salamandra) inferred from discordance of genetic and morphological traits. Evolution 57:129–143
Gomez-Mestre I, Pyron RA, Wiens JJ (2012) Phylogenetic analyses reveal unexpected patterns in the evolution of reproductive modes in frogs. Evolution 66:3687–3700
Goodwin NB, Dulvy NK, Reynolds JD (2002) Life-history correlates of the evolution of live bearing in fishes. Philos Trans R Soc B 357:259–267
Gower D, Giri V, Dharne MS, Shouche YS (2008) Frequency of independent origins of viviparity among caecilians (Gymnophiona): evidence from the first ‘live-bearing’ Asian amphibian. J Evol Biol 21:1220–1226
Greven H (1998) Survey of the oviduct of salamandrids with special reference to the viviparous species. J Exp Zool 282:507–525
Greven H (2011) Maternal adaptations to reproductive modes in amphibians. In: Norris P, Lopez K (eds) Hormones and reproduction of vertebrates, volume 2, amphibians. Elsevier, USA, pp 117–141
Harrison RM (2001) Reproduction in mammals: general overview. In: eLS. Wiley, Chichester. doi:10.1038/npg.els.0001855
Heulin B, Osenegg K, Lebouvier M (1991) Timing of embryonic development and birth dates in oviparous and viviparous strains of Lacerta vivipara: testing the predictions of an evolutionary hypothesis. Acta Oecol 12:517–528
Heulin B, Osenegg-Leconte K, Michel D (1997) Demography of a bimodal reproductive species of lizard (Lacerta vivipara): survival and density characteristics of oviparous populations. Herpetologica 53:432–444
Hodges WL (2004) Evolution of viviparity in horned lizards (Phrynosoma): testing the cold-climate hypothesis. J Evol Biol 17:1230–1237
Jamieson BGM (2009) Reproductive biology and phylogeny of fishes (agnathans and bony fishes). Science Publishers, Enfield, NH
Janvier P (2001) Agnatha (lampreys, hagfishes, ostracoderms). In: eLS. Wiley, Chichester. doi:10.1038/npg.els.0001532
Joly J (1986) La réproduction de la salamandre terrestre (Salamandra salamandra L.). In: Grasse PP, Delsol M (eds) Traité de Zologie. Amphibiens, vol 14. Masson, Paris, pp 471–486
Kaplan RH, Cooper WS (1984) The evolution of developmental plasticity in reproductive characteristics: an application of the ‘adaptive coin-flipping’ principle. Am Nat 123:393–410
Kopp M, Baur B (2000) Intra- and inter-litter variation in life-history traits in a population of fire salamanders (Salamandra salamandra terrestris). J Zool 25:231–236
Kupfer A, Müller H, Antoniazzi MM, Jared C, Greven H, Nussbaum RA, Wilkinson M (2006) Parental investment by skin feeding in a caecilian amphibian. Nature 440:926–929
Lambert SM, Wiens JJ (2013) Evolution of viviparity: a phylogenetic test of the cold-climate hypothesis in phrynosomatid lizards. Evolution 67:2614–2630
Lomolino MV (2005) Body size evolution in insular vertebrates: generality of the island rule. J Biogeogr 32:1683–1699
Pyron RA, Burbrink FT (2014) Early origin of viviparity and multiple reversions to oviparity in squamate reptiles. Ecol Lett 17:13–21
Qualls CP, Shine R (1998) Costs of reproduction in conspecific oviparous and viviparous lizards, Lerista bouganvillii. Oikos 82:539–551
Rebelo R, Leclair MH (2003) Differences in size at birth and brood size among Portuguese populations of the fire salamander, Salamandra salamandra. Herpetol J 13:179–188
Rodríguez-Díaz T, Braña F (2012) Altitudinal variation in egg retention and rates of embryonic development in oviparous Zootoca vivipara fits predictions from the cold-climate model of the evolution of viviparity. J Evol Biol 25:1877–1887
Roitberg ES, Kuranova VN, Bulakhova NA, Orlova VF, Eplanova GV, Zinenko OI, Shamgunova RR, Hofmann S, Yakolev VA (2012) Variation of reproductive traits and female boy-size in the most widely-ranging terrestrial reptile: testing the effects of reproductive mode, lineage, and climate. Evol Biol 40:420–438
Salvador A, García-París M (2001) Anfibios Españoles. Identificación, historia Natural y Distribución. Canseco Editores, S. L, Talavera de la Reina, Spain
Semlitsch RD, Schmiedehausen S (1994) Parental contributions to variation in hatchling size and its relationship to growth and metamorphosis in tadpoles of Rana lessonae and Rana esculenta. Copeia 1994:406–412
Shine R (1985) The evolution of viviparity in reptiles: an ecological analysis. In: Gans C, Billet F (eds) Biology of the reptilia. Wiley, New York, pp 605–694
Shine R (2014) Evolution of an evolutionary hypothesis: a history of changing ideas about the adaptive significance of viviparity in reptiles. J Herpetol 48:147–161
Statsoft Inc (2010) STATISTICA 10.0. Statsoft, Inc, Tulsa, OK
Stewart JR (2003) Fetal nutrition in lecitotrophic reptiles: toward a comprehensive model for evolution of viviparity and placentation. J Morphol 274:824–843
Thiesmeier B, Haker K (1990) Salamandra salamandra bernardezi Wolterstorff 1928 aus Oviedo, Spanien, nebst Bermerkungen zu Viviparie in der Gattung Salamandra. Salamandra 26:140–154
Travis J, Emerson SB, Blouin M (1987) A quantitative genetic analysis of larval life-history traits in Hyla crucifer. Evolution 41:45–156
Uotila U, Crespo-Díaz A, Sanz-Azkue et al (2013) Variation in the reproductive strategies of fire salamander populations in the province of Gipuzkoa (Basque Country). Munibe 61:91–101
Veith M, Steinfartz S, Zardoya R et al (1998) A molecular phylogeny of ‘true’ salamanders (family Salamandridae) and the evolution of terrestriality of reproductive modes. J Zool Syst Evol Res 3:7–16
Velo-Antón G (2008) Presencia de Salamandra salamandra en la isla de Tambo (Rías Bajas, Pontevedra). Bol Asoc Esp Herp 19:61–62
Velo-Antón G, García-París M, Galán P et al (2007) The evolution of viviparity in Holocene islands: ecological adaptation versus phylogenetic descent along the transition from aquatic to terrestrial environments. J Zool Syst Evol Res 45:345–352
Velo-Antón G, Zamudio KR, Cordero-Rivera A (2012) Genetic drift and rapid evolution of viviparity in insular fire salamanders (Salamandra salamandra). Heredity 108:410–418
Wade MJ, Shuster SM (2002) The evolution of parental care in the context o sexual selection: a critical reassessment of parental investment theory. Am Nat 160:285–292
Wake MH (2002) Viviparity and oviparity. In: Pagel MD (ed) Encyclopedia of evolution, vol 2. Oxford University Press, New York, pp 1141–1143
Wake MH (2003) Reproductive modes, ontogenies, and the evolution of body forms. Anim Biol 53:209–223
Wake MH (2014) Fetal adaptations for viviparity in amphibians. J Morphol. doi:10.1002/jmor.20271
Wells KD (2007) The ecology and behavior of amphibians. The University of Chicago Press, Chicago
Wourms JP (1981) Viviparity: the maternal–fetal relationship in fishes. Am Zool 21:473–515
Acknowledgments
We are deeply thankful to M. Casal Nantes for her generous help during field-work campaigns. We thank the employees of the National Park for facilitating our trips to the island. Fieldwork for obtaining tissue samples was done with the corresponding permits from regional administration (Galicia, Ref. 1653/2009, 014/2011, 546/2012). We also thank M. Modrell, the associated editor Martin Reichard and two anonymous reviewers for all their comments and suggestions, which have certainly improved the manuscript. G.V.-A. and X.S. are supported by Fundação para a Ciência e Tecnologia (SFRH/BPD/74834/2010 and SFRH/BPD/73176/2010, respectively). D.B. was partially supported by a JAE-DOC fellowship from the CSIC under the program “Junta para la Ampliación de Estudios” co-financed by the European Social Fund (ESF). This study was partially supported by Grants from the Organismo Autónomo Parques Nacionales (Grant 072B/2002) and by Fundação para a Ciência e a Tecnologia (FCT: PTDC/BIA-EVF/3036/2012) through EU Programme COMPETE, and by project “Biodiversity, Ecology and Global Change” co-financed by North Portugal Regional Operational Programme 2007/2013 (ON.2—O Novo Norte), under the National Strategic Reference Framework (NSRF), through the European Regional Development Fund (ERDF). This research also received support from the SYNTHESYS Project http://www.synthesys.info/ which is financed by European Community Research Infrastructure Action under the FP7 “Capacities” Programme at the Museo Museo Nacional de Ciencias Naturales (CSIC) (Ref: ES-TAF-1987).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 2.
Rights and permissions
About this article
Cite this article
Velo-Antón, G., Santos, X., Sanmartín-Villar, I. et al. Intraspecific variation in clutch size and maternal investment in pueriparous and larviparous Salamandra salamandra females. Evol Ecol 29, 185–204 (2015). https://doi.org/10.1007/s10682-014-9720-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-014-9720-0