Abstract
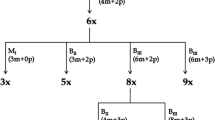
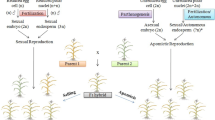
One hundred and sixty accessions representing global germplasm of guinea grass (Panicum maximum Jacq.), an important apomictic (aposporous) fodder crop, were subjected to study on reproductive diversity in apomictic seed development utilizing ovule clearing and flow cytometric seed screen (FCSS). Single seed FCSS of selected 14 tetraploid and five hexaploid lines demonstrated uncoupling between the three apomixis components, viz. apomeiosis, parthenogenesis and functional endosperm development, in natural as well as experimental populations, though it differed across ploidy levels and genotypes. Reconstruction of reproductive pathways yielded a total of eight different pathways of seed development, arising by uncoupling/recombination between apomixis components. Amongst these, two pathways involving modifications in embryo-sac (ES) (presence of two polar nuclei in aposporous ES that fuse prior to fertilization) and fertilization process (fusion of only one polar nucleus in a sexual ES) have been reported for the first time. Some of the combinations, such as MI (haploids arising from parthenogenetic development of reduced egg cell), were found viable only in hexaploid background. Germplasm lines with higher expression of individual components were also identified. These components (including autonomous endosperm development) were also experimentally partitioned in hexaploid progenies (derived from a tetraploid parent viz. accession IG 04-164) that showed segregation in their reproductive capacities, and are reported for the first time. Occurrence of several apomixis recombinants (phenotypic) in guinea grass lines suggested their hybrid origin, favors a multigene model for apomixis, with their penetrance affected by modifiers and epigenetic mechanisms, in contrast to earlier reports of single locus control. Implications of partitioning components on apomixis research are discussed.


Similar content being viewed by others
References
Albertini E, Porceddu A, Ferranti F, Reale L, Barcaccia G, Romano B, Falcinelli M (2001) Apospory and parthenogenesis may be uncoupled in Poa pratensis: a cytological investigation. Sex Plant Reprod 14:213–217
Anonymous (2007) Apomixis—guinea grass. Annual report 2006–2007. Indian Grassland and Fodder Research Institute, Jhansi, India
Barcaccia G, Arzenton F, Sharbel TF, Varotto S, Parrini P, Lucchin M (2006) Genetic diversity and reproductive biology in ecotypes of the facultative apomict Hypericum perforatum L. Heredity 96:322–334
Bicknell RA, Koltunow A (2004) Understanding apomixis: recent advances and remaining conundrums. Plant Cell 16:S228–S245
Bicknell RA, Lambie SC, Butler RC (2003) Quantification of progeny classes in two facultatively apomictic accessions of Hieracium. Hereditas 138:11–20
Burson BL, Hussey MA, Actkinson JM, Shafer GS (2002) Effect of pollination time on the frequency of 2n + n fertilization in apomictic buffelgrass. Crop Sci 42:1075–1080
Chen LZ, Kozono T (1994a) Cytological evidence of seed-forming embryo development in polyembryonic ovules of facultatively apomictic guinea grass (Panicum maximum Jacq.). Cytologia 59:351–359
Chen LZ, Kozono T (1994b) Cytology and quantitative analysis of aposporous embryo sac development in guinea grass. Cytologia 59:253–260
Combes D (1975) Polymorphisme et mode de reproduction dans la section des Maximae du genre Panicum (Graminees) en Afrique. Coll Menoires ORSTOM, Paris 77:1–100
de Wet JMJ (1968) Diploid-tetraploid-haploid cycles and the origin of variability in Dicanthium agamospecies. Evolution 22:394–397
Dujardin M, Hanna WW (1986) An apomictic polyhaploid obtained from a pearl millet × Pennisetum squamulatum apomictic interspecific hybrid. Theor Appl Genet 72:33–36
Ebina M, Nakagawa H, Yamamoto T, Araya H, Tsuruta S, Takahara M, Nakajima K (2005) Co-segregation of AFLP and RAPD markers to apospory in guinea grass (Panicum maximum Jacq.). Japan Soc Grassl Sci 51:71–78
Grimanelli D, Harnandez M, Perotti E, Savidan Y (1997) Dosage effects in the endosperm of diplosporous apomict Tripsacum (Poaceae). Sex Plant Reprod 10:279–282
Jain A, Zadoo SN, Roy AK, Kaushal P, Malaviya DR (2003) Meiotic system and probable basic chromosome number of Panicum maximum Jacq. accessions. Cytologia 68:7–13
Jain A, Roy AK, Kaushal P, Malaviya DR, Zadoo SN (2006) Isozyme banding pattern and estimation of genetic diversity among guinea grass germplasm. Genet Resour Crop Evol 53:339–347
Kaushal P, Malaviya DR, Singh KK (1999) Evaluation of exotic accessions of guinea grass (Panicum maximum Jacq.). Ind J Plant Genet Resour 12:215–218
Kaushal P, Malaviya DR, Roy AK (2004) Prospects for breeding apomictic rice: a reassessment. Curr Sci 87:292–296
Kaushal P, Roy AK, Khare A, Malaviya DR, Zadoo SN, Choubey RN (2007) Crossability and characterization of interspecific hybrids between sexual Pennisetum glaucum (pearl millet) and a new cytotype (2n = 56) of apomictic P. squamulatum. Cytologia 72:111–118
Koltunow AM, Grossniklaus U (2003) Apomixis: a developmental perspective. Annu Rev Plant Biol 54:547–574
Koltunow AM, Johnson SD, Bicknell RA (2000) Apomixis is not developmentally conserved in related, genetically characterized Hieracium plants of varying ploidy. Sex Plant Reprod 12:253–266
Malaviya DR (1998) Evaluation of Panicum maximum lines for sustained productivity. Range Manage Agroforestry 19:126–132
Malaviya DR, Kaushal P (2005) IGFRI Guinea grass germplasm catalogue. Indian Grassland and Fodder Research Institute, Jhansi, pp 26
Martelotto LG, Ortiz JPA, Stein J, Espinoza F, Quarin CL, Pessino SC (2007) Genome rearrangements derived from autopolyploidization in Paspalum sp. Plant Sci 172:970–977
Matzk F (1991) A novel approach to differentiated embryos in the absence of endosperm. Sex Plant Reprod 4:88–94
Matzk F, Meister A, Schubert I (2000) An efficient screen for reproductive pathways using mature seeds of monocots and dicots. Plant J 21:97–108
Matzk F, Meister A, Brutovska R, Schubert I (2001) Reconstruction of reproductive diversity in Hypericum perforatum L. opens novel strategies to manage apomixis. Plant J 26:275–282
Matzk F, Prodanovic S, Baumlein H, Schubert I (2005) The inheritance of apomixis in Poa pratensis confirms a five locus model with differences in gene expressivity and penetrance. Plant Cell 17:13–24
Mecchia MA, Ochogavia A, Selva JP, Laspina N, Felitti S, Martelloto LG, Spangenberg G, Echenique V, Pessino SC (2007) Genome polymorphisms and gene differential expression in a ‘back-and-forth’ ploidy altered series of weeping lovegrass (Eragrotis curvula). J Plant Physiol 164:1051–1061
Morgan RN, Ozias-Akins P, Hanna WW (1998) Seed set in an apomictic BC3 pearl millet. Int J Plant Sci 159:87–89
Nakagawa H, Hanna WW (1990) Morphology, origin and cytogenetics of a 48-chromosome Panicum maximum. Cytologia 55:471–474
Nogler GA (1984) Gametophytic apomixis. In: Johri BM (ed) Embryology of angioperms. Springer, Berlin, pp 475–518
Noyes RD (2005) Inheritance of apomeiosis (diplospory) in fleabanes (Erigeron, Asteraceae). Heredity 94:193–198
Noyes RD (2006) Apomixis via recombination in genome regions for apomeiosis (diplospory) and parthenogenesis in Erigeron. Sex Plant Reprod 19:7–18
Noyes RD, Rieseberg LH (2000) Two independent loci control agamospermy (apomixis) in the triploid flowering plant Erigeron annus. Genetics 155:379–390
Ozias-Akins P (2006) Apomixis: developmental characteristics and genetics. Crit Rev Plant Sci 25:199–214
Ozias-Akins P, vanDijk PJ (2007) Mendelian genetics of apomixis in plants. Annu Rev Genet 41:509–537
Reddy PS, d’Cruz R (1969) Mechanism of apomixis in Dicanthium annulatum (Forskk) Stapf. Bot Gaz 130:71–79
Roche D, Hanna W, Ozias-Akins P (2001) Is supernumerary chromatin involved in gametophytic apomixis of polyploid plants? Sex Plant Reprod 13:343–349
Rodrigues JCM, Koltunow AMG (2005) Epigenetic aspects of sexual and asexual seed development. Acta Biol Cracoviensia Ser Bot 47:37–49
Rutihauser A (1948) Pseudogamie und polymorphie in der gattung Potentilla. Arch Julius Stiftung 23:267–424
Savidan Y (1980) Chromosomal and embryological analyses in sexual × apomictic hybrids of Panicum maximum Jacq. Theor Appl Genet 57:153–156
Savidan Y (1981) Genetics and utilization of apomixis for the improvement of guinea grass (Panicum maximum Jacq.) In: Proc XIV Intl Grassl Congr, Lexington, pp 182–184
Savidan Y (2000) Apomixis: genetics and breeding. Plant Breed Rev 18:13–85
Savidan Y, Pernes J (1982) Diploid-tetraploid-dihaploid cycles and the evolution of Panicum maximum Jacq. Evolution 36:596–600
Savidan YH, Jank L, Costa LCG, doValle CB (1989) Breeding Panicum maximum in Brazil. 1. Genetic resources, modes of reproduction and breeding procedures. Euphytica 41:107–112
Schranz ME, Kantama L, de Jong H, Mitchell-Olds T (2006) Asexual reproduction in a close relative of Arabidopsis: a genetic investigation of apomixis in Boechera (Brassicaceae). New Phytol 171:425–438
vanDijk PJ, van Baarlen P, deJong H (2003) The occurrence of phenotypically complementary apomixis-recombinants in crosses between sexual and apomictic dandelions (Taraxacum officinale). Sex Plant Reprod 16:71–76
Warmke HE (1954) Apomixis in Panicum maximum. Am J Bot 41:5–11
Wieners RR, Fei S, Johnson RC (2006) Characterization of a USDA Kentucky bluegrass (Poa pratensis L.) core collection for reproductive mode and DNA content by flow cytometry. Genet Resour Crop Evol 53:1531–1541
Yao J-L, Zhou Y, Hu C-G (2007) Apomixis in Eulaliopsis binata: characterization of reproductive mode and endosperm development. Sex Plant Reprod 20:151–158
Young BA, Sherwood RT, Bashaw EC (1979) Cleared-pistil and thick sectioning techniques for detecting aposporous apomixis in grasses. Can J Bot 57:1668–1672
Zavesky L, Jarolimova V, Stepanek J (2007) Apomixis in Taraxacum paludosum (section Palustria, Asteraceae): recombinations of apomixis elements in inter-sectional crosses. Plant Syst Evol 265:147–163
Acknowledgements
Germplasm lines from various sources are thankfully acknowledged. Support by Department of Science and Technology (DST-Fast Track Scheme-Grant SR/L-93/2003) and by Indian Council of Agricultural Research, India, (AP Cess Scheme-Grant 3030522031) is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaushal, P., Malaviya, D.R., Roy, A.K. et al. Reproductive pathways of seed development in apomictic guinea grass (Panicum maximum Jacq.) reveal uncoupling of apomixis components. Euphytica 164, 81–92 (2008). https://doi.org/10.1007/s10681-008-9650-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-008-9650-4