Abstract
The effects of the long-term contamination of water reservoirs with mine effluents were investigated at an abandoned mine site in Upper Silesia, southern Poland. The studies covered metal content and mobility in bottom sediments as well as water chemistry in relation to the content of metals in selected macrophytes and their physiology and the composition of phyto- and zooplankton communities. Although it is 40 years since mining ceased, reservoir sediments are still heavily contaminated with cadmium, zinc and lead with concentrations (mg/kg), which vary roughly between 130–340, 10,000–50,000 and 4,000–12,000, respectively. About 50–80 % of these elements are associated with the reducible phase, and only a small percentage, <10 %, is present in the most mobile exchangeable phase. Despite the high total metal concentration in sediments, their content in the submerged plants Myriophyllum spicatum and the emerged plants Phragmites australis was low. The observed effects of heavy metal contamination on photosynthetic activity in the leaves of P. australis were negligible, whereas those in M. spicatum show up only as a difference in the distribution of photosynthetic activity in leaves of different ages, which seems to be related to the very good water quality and to the generally small concentrations of metals in pond water. The physicochemical properties of water also seem to control the presence of planktonic species more than does sediment contamination. However, a shift toward groups of species known to be more resistant to heavy metals (diatoms, green algae and Rotifera) indicates some adaptative changes related to the long-lasting contamination of ponds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Very high concentrations of heavy metals occur close to metal mines and the associated ore processing plants long after mining ceases. Contamination may result from the chemical and physical degradation of exposed waste heaps, tailing ponds or the remnants of ore bodies. The oxidation of sulphide wastes or dust blowing is known to affect bare soils mostly in dry lands, whereas in moderate or wet climates, sheet and gulley erosion of waste heaps decreases with time as a result of spontaneous plant encroachment and the leaching of metalliferous spoils begins to prevail (Wilson and Pyatt 2007; Rodriguez et al. 2009). The hampering of erosion by vegetation at mine site is, however, affected by heavy metals, and the extent of this depends on the plant species, the metal concentration in the substratum and its bioavailability (Lim et al. 2008). High metal concentrations may prevent the growth of protective vegetation cover because metal uptake affects the relative composition (Alvarez et al. 2003), leading to the appearance or even domination of plants (called hyperaccumulators), which tolerate high metal content (Escarre et al. 2011).
Former mine sites contaminate also the aquatic environment either directly from the drainage of adits (Younger 1997), surface runoff and the spillway of tailing ponds (Marques et al. 2001) or groundwater seepage (Mighanetara et al. 2009). Moreover, metals discharged with mine waters during mine operation precipitate on the river bed or accumulate with fine grained sediments. In small streams with low discharge variability, sediment-associated heavy metals, persisting for tens of years after the end of mining or smelting operations (Ciszewski 2004), are mobilised, resulting in the long-term contamination of surface waters (Hutchinson and Rothwell 2008; Byrne et al. 2010). In addition, the small Matylda stream in southern Poland was found to be contaminated by former mine operations with Zn, Pb and Cd concentrations in bed sediments exceeding 4 %, 1.5 % and 400 mg/kg, respectively (Ciszewski et al. 2012). It received mine waters for over 100 years from a lead–zinc mine. The mine was established in 1850 and pumped up to 0.5–1.0 m3/s of mine water into the naturally meandering small stream. In 1926, the mine was expanded and the upper section of the Matylda stream was converted into a trapezoidal, straight channel. The operation of the mine finished in 1972, and since that year, the Matylda catchment is drained naturally by a stream whose discharge is of dozen of litres per second.
Channelisation of the stream in the 1920s and 1930s was accompanied by the establishment of a cascade of fishponds in the middle section of the catchment, supplied partially with mine waters discharged to the stream. At present, the fishponds still receive waters from the Matylda stream and during high water stages from a periodic side tributary. The fishponds are partially filled with sediments originating from the mine, which may be a source of heavy metals. It is particularly interesting in the context of using the ponds for recreational angling and an estimation of the potential danger for anglers based on the metal content in sediments and waters, as well as in studying the contamination effects on aquatic plants and phyto- and zooplankton communities.
Ponds and lakes in mining areas are known to be long-term sinks for heavy metals, which affect aquatic biota in a way not proportional to degree of sediment contamination (Lukin et al. 2003). The effects are considered to depend on numerous factors such as water and sediment properties, the shape and depth of the reservoir or biosystem productivity (Suchanek et al. 2008). Generally, the content of heavy metals in aquatic macrophytes affected by metal mining and smelting is higher than in reference areas and can change from year to year and with changes of pH, redox potential, temperature and the salinity of water, which control metal uptake by submersed plants (Franzin and McFarlane 1980; Srivastava et al. 2008). Aquatic macrophytes can take up elements from the water column via leaves and from sediment via roots (Kelly and Pinder 1996; Lewander et al. 1996), which can be in large amounts (Sánchez et al. 1998; Lesage et al. 2007; Li et al. 2010). These plants differ both in their capacity to take up and transfer metals to above-ground parts and in the relative proportion of direct metal adsorption from water as opposed to uptake from sediments, which can obscure the effects of sediment metal contamination in itself (Welsh and Denny 1980). High concentrations of heavy metals in the water environment may cause several adverse effects in a plant. Accordingly, a lot of field and experimental studies on the pathways of metal uptake and translocation in macrophytes have already been conducted (Crowder 1991; Jackson et al. 1996; Lewander et al. 1996; Shinmachi et al. 2003; Dogan and Saygideger 2004; Weis et al. 2004; Markich et al. 2006; Mishra et al. 2008).
Photosyntesis is one of the most sensitive targets of heavy metals in plant cells. Different effects can be observed in both the structure and functioning of the photosynthetic apparatus (Bragato et al. 2006). The photosynthetic pigment–protein complex, which shows a special susceptibility to heavy metal excess is photosystem II (PSII), where many sites of different heavy metal action have been described (Sarvari 2005). The process of the photoactivation of PSII seems to be, under prolonged heavy metal stress, sensitive to concentrations of at least one order lower than those of any other process in the bioenergetic machinery of the plant cell (Faller et al. 2005). That is why photosynthesis and particularly PSII activity are often used in the investigation of heavy metal stress in plants. The most often used parameter of photosynthetic activity is F V/F M—a maximum quantum efficiency of primary photochemistry in PSII, measured by the chlorophyll a fluorescence method. The measurement of F V/F M is easy, very fast and non-destructive, so changes in this parameter are used in many investigations as a measure of physiological state of the plant. It is worth noting that F V/F M has shown a low sensitivity to the action of heavy metals, which is why the use of the more sensitive parameters obtained by more sophisticated methods of chlorophyll measurements have been recently proposed (Lichtenthaler et al. 2005). Among these, a particularly useful one seems to be the analysis of the fluorescence kinetics induction curve (recorded by a chlorophyll fluorometer during standard F V/F M measurement) what is known as the OJIP test (Strasser et al. 2004). It can be used to describe the changes in photosynthetic energy flow under particular stress conditions. One parameter obtained from an OJIP test, S M, which reflects the multiple turnover of Q A reduction during fluorescence transient from F O to F M, was used here as an indirect measure of the secondary electron acceptor pool of PSII.
Long-term reservoir contamination by mining-related effluents also affects algae and zooplankton communities for decades after the end of mining (Salonen et al. 2006). Changes in their biomass, species richness and inter-species relationships being shifted towards the domination of less sensitive species may be well correlated to metal contamination, although changes in metal content are usually related also to changes in water chemistry, particularly acidity (Cattaneo et al. 2004). That is why numerous studies have indicated that some algae and zooplanktonic organisms may tolerate high concentrations of heavy metals and do not therefore respond to the contamination of aquatic environment by heavy metals (Shubert et al. 2001).
The aim of the present studies is to assess the effects of the long-term contamination of water reservoirs by mine effluents at an abandoned mine site by studying Cu, Cd, Pb, Zn, Mn, and Fe contents and their mobility in bottom sediments. It also aims to study water chemistry in relation to the content of these metals in selected macrophytes, along with their physiology and the composition of phyto- and zooplankton communities.
Materials and methods
Study area
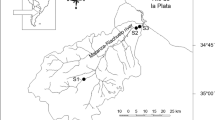
The catchment of the Matylda stream studied is located in the lowest part of the Silesian Upland, in southern Poland (Fig. 1). Its surface covers only a dozen square kilometers, and it drains ore-bearing Triassic dolomites, formerly excavated in the mine in its upper reaches to a depth of about 100 m. The entire catchment is filled with fluvioglacial sandy deposits, which cover the dolomites with a layer of a dozen or so metres on average. The catchment is overgrown with forest, and in the upper reaches, there is the built-up area of Chrzanów city and the partially reclaimed area of the lead–zinc mine Matylda. Although the mine had been operated since the mid-nineteenth century, its exploitation in the years 1953–1972 was several tens of times larger than during the previous period. Since the closure of the mine the Matylda stream has been moderately contaminated with storm waters and some municipal sewage from Chrzanów city (40,000 inhabitants) situated in the upper section of the catchment.
Research area and sampling points: a dykes and roads; b dykes; c bottom sediment sampling points; d points of water, aquatic plants, and planctonic surveys. RP Reference pond, SP small pond, MP middle pond, LP large pond; I main roads, II roads, III railways, IV forest tracks, V fish ponds, VI forests, VII closed Matylda mine, VIII, built-up area
The cascade of three ponds constructed in the middle part of the catchment consists of an upper small pond (SP), a medium middle pond (MP) and the largest lower pond (LP; Fig. 1). Water from the Matylda mine has been fed directly to the upper pond and flowed to the outlet at the lowest end of the large pond. Moreover, the smallest pond has also been directly fed also by waters of a small side, 2 km of long, stream whose discharge varies from zero, mostly in the autumn–winter season to several litres per second in the warm season. This stream that drain shallow wetland is the only tributary for another two ponds nearby; one of these ponds was studied here as a reference pond (RP; Fig. 1). The reference and the largest pond are about 2 m deep, whereas the small and middle pond is filled with sediments to a depth of 0.5–1.5 m. The surface of the largest pond is 15 ha, whereas the small and middle ponds are about 1 and 4 ha, respectively. All the investigated ponds are used for recreational angling with fish introduced every 2 months, three to four times per year during the warm season.
Water and sediment sampling and analyses
Samples of water were collected monthly from the bank and from the middle part (2 points) of each cascade pond and from the middle part (1 point) of the reference pond for the period of 1 year. Bottom sediment samples were taken in two to three right-side cross-sections from each cascade pond and at 2 points from the reference pond (Fig. 1). In total, 24 sediment grab samples were collected from the surface 0–10 cm layer of the ponds.
Conductivity and pH were determined by means of WTW apparatus. Water samples were filtered through 0.45-μm pore-sized syringe filters into a polyethylene container. Inorganic anions Cl−, NO3 −, SO4 −2, PO4 −3, CO3 −2 and cations Ca2+, Mg2+, Na+ and K+ were analysed within 48 h using ion chromatography (DIONEX ICS 1000 and IC DX 320). For metal analysis, samples of water were additionally acidified to a pH <2 with ultrapure HNO3. Concentrations of Zn, Cd, Pb, Cu, Fe and Mn were measured with an inductively coupled plasma mass spectrometer (Perkin Elmer ELAN 6100).
From each sample of sediment a silt-clay fraction (0.063 mm) was wet-separated. Metals (Cu, Cd, Pb, Zn, Mn and Fe) were brought into solution in Teflon bombs using a microwave technique (10 cm3 of 65 % HNO3 and 2 cm3 of 30 % H2O2). In the samples, the speciation of heavy metals was determined using a standard BCR procedure to extract heavy metals in three operationally defined geochemical phases (Rauret et al. 1999): (1) exchangeable and bound to carbonates; (2) reducible, bound to iron and manganese oxides; and (3) oxidisable, bound to organic and sulphide compounds. Additionally, one more (4) residual chemical fraction was extracted using 65 % HNO3. The concentrations of metals were determined using a flame AA spectrometer. Standard reference materials (Canadian waters Hamilton-20) were employed to determine the accuracy of anion analyses, whereas metal analyses were performed according to the standard certified quality control procedure (PN-EN ISO 17294-1:2007).
Sampling and analyses of aquatic macrophytes
Two species of aquatic macrophytes: Myriophyllum spicatum L. (submerged) and Phragmites australis (Cav., Trin. ex Steud) were sampled to analyse Cd, Pb, Cu and Zn concentrations. M. spicatum was collected from the central part of the small, middle and lower pond and P. australis from along the northern bank of the all ponds once in summer season (5 August 2009; Fig. 1).
In the laboratory, the macrophytes were first gently cleaned with tap and then with redistilled water. Then, shoots of M. spicatum as well as stems, leaves and roots of P. australis were separated for further analysis. Samples of macrophytes (three subsamples from each species) were dried at 105 °C for 48 h and homogenised using the Planetary Mill ‘Pulverisette 5’ with Teflon grinding balls. Then, they were digested with concentrated HNO3 and HCl using a microwave technique (Speed Wave, Berghof). Concentrations of heavy metals in macrophytes were analysed with the atomic absorption spectroscopy method using an atomic absorption spectrophotometer (Varian-20). Reference Material (BCR®-60, aquatic plant, Lagarosiphon major) was used to determine the analytical accuracy. Comparisons of measured and certified values of analytical standard concentrations are given in Table 1.
Photosynthetic activity was compared in two species of macrophytes: P. australis and M. spicatum, which were present in all ponds. Additional measurements were performed on Ceratophyllum demersum present in SP only. Measurements of chlorophyll a fluorescence induction kinetics (Kautsky curves) in P. australis were made in situ, each approximately 20 m, along E–W transect (parallel to the main direction of the stream) on the northern bank of SP, MP and LP and along N–S transect on the western bank of the RP between 10 a.m. and 12:30 p.m. on May 14th, 2010. It was warm (25–27 °C) and sunny day (500–700 μmol m−2 s−1 of photons PhAR) with no clouds and practically without wind. Measurements were performed on the middle part of the second youngest fully developed leaf using a Handy-PEA (Hansatech, UK) chlorophyll fluorometer with standard procedure, at an excitation light intensity of 3,500 μmol m−2 s−1. The measured part of a leaf was darkened for at least 20 min. Records were then analysed using PEA-Plus software, dedicated to the fluorometer.
The imaging of the photosynthetic activity of Myriophyllum and Ceratophyllum shoots was performed in the laboratory on freshly harvested plants kept in natural water from the habitat. Measurements of about 15 cm apical ends of shoots (about 20 whorls + shoot tip) submerged in its natural water were carried out not later than 48 h after plant collection, using the Open FluorCam FC 800-O chlorophyll fluorescence imaging system (Photon System Instruments, Czech Rep.). The basic fluorescence level was measured at 0.1 μmol m−2 s−1 of photons and following Kautsky induction, kinetics of fluorescence was registered at 200 μmol m−2 s−1 of photons. Both lights used were red, with λ max = 635 nm. For an analysis of results, dedicated software was used.
Phyto- and zooplankton sampling and analyses
Samples for phyto- and zooplankton were taken from the central point of each pond (Fig. 1). Each sample consisted of organisms from the entire water column. For taxonomic identification and quantitative analyses, samples for phyto- and zooplankton were collected using a 5-L Ruttner sampler. In the field, 30-L of water samples (six replicate, 5 L samples) were concentrated with a 10-μm plankton net for phytoplankton and a 50-μm plankton net for zooplankton. Samples for phytoplankton were immediately fixed with Lugol’s solution, and 4 % formalin was used to fix the zooplankton. For the identification and counting of phytoplankton species, a modified chamber of 0.4 mm height and 22 mm in diameter was used. All microscopic analyses of phytoplankton were done under a Zeiss Jenaval microscope. Quantitative analyses were conducted according to Lund et al. (1958). For the keys used for the taxonomical analyses of phytoplankton, see e.g. Wilk-Wozniak (2009).
For the identification and counting of zooplankton species, five replicate sub-samples were analysed microscopically (×100 or ×200) in a chamber of 0.5 ml of volume. Taxonomic analyses of zooplankton (rotifer, copepod and cladoceran taxa) were conducted using keys according to Pociecha et al. (2010). Quantitative samples were prepared by filtering 30 L of water and reducing the sample volume to 50 mL.
Results and discussion
Water chemistry
The values of almost all parameters investigated in water decrease from the small to the large pond (Table 2). The rate of changes is to some extent reflected by a progressive decrease in conductance and dry residue. However, for nitrates, ammonia and calcium, the change between the most polluted small and medium ponds is much more rapid. The opposite trend is observed for pH, which rises slightly down the valley and for biological oxygen demand (BOD), which rises between SP and MP and then drops in LP. Water is well oxygenated with a constant oxygen content of about 9 mg/L. In contrast to the generally small changes in the above-mentioned parameters, the content of heavy metals is much more variable. The content is markedly higher in the small pond than in the medium and large ponds. It drops by about two to three times for cadmium and iron, four to five times for lead and manganese and almost 30 times for copper. The decrease in the metal content between the lower two ponds is smaller, while the content of copper and lead even rises. The values of these parameters in the cascade of ponds are generally higher than in the reference pond with the exception of dissolved carbon, phosphates and cadmium, which are higher or comparable to that in the small pond.
The highest content of macroions in the small pond is related to the direct supply of moderately contaminated waters from Matylda. Pond waters are mixed there with weakly mineralised waters from the side tributary and with relatively clean groundwater, which easily percolate through the sandy sediments, which fill the valley bottom (Aleksander-Kwaterczak and Ciszewski 2012). Moreover, the abundant aquatic plants and blooming algae play an important role in the transformation of water chemistry in ponds (Wilk-Wozniak et al. 2011). Particularly, the blooming of summer algae is responsible for rise in pH, which even reaches 9.7 in the large pond in June. Losses of nitrate and of dissolved heavy metals (especially Cu) in the water of the cascade ponds is probably mainly due to their uptake and adsorption by water plants particularly abundant in SP (Mazurkiewicz-Boroń 2002). The high amount of ammonia indicates that processes of organic matter decomposition are the most intensive in the SP. In addition, the accumulation of dead organisms in the bottom undoubtedly plays an important role as a sink for heavy metals. Metals may be adsorbed onto the suspended cells of dead algae and the surface of macrophytes or temporarily co-precipitate with iron compounds. It is characteristic of well-oxygenated waters with high pH to favour manganese and iron oxide precipitation and accumulation in the bottom (Jackson and Bistricki 1995). The phenomenon is suggested here by the large drop in iron and manganese content in water between the small and medium ponds. The reduction in metal content in water also benefits from pH over neutral, particularly during the vegetation season and quite high carbonate content, which encourages the formation of the most widespread heavy metals bonds in bottom sediments.
Sediment contamination and metal mobility
Concentrations of heavy metals in bottom sediments vary markedly between the investigated ponds (Table 3). The largest average concentrations of each element occur in the small upper pond (SP) situated at the inflow of waters from the Matylda stream. Cadmium content varies roughly between 130 and 340 mg/kg, zinc content between 10,000 and 50,000 mg/kg and lead with the range of 4,000–12,000 mg/kg. These concentrations are several hundred times higher than in forest soils at a depth of 0.8–1.0 m in this area, which can be considered as the local geochemical background (Pasieczna 2011). This is indeed typical of high geoaccumulation patterns observed in other parts of the world facing similar environmental pressures (Nikolaidis et al. 2010). The fall in median values reflects a decrease in heavy metal pollution in the cascade of ponds. Its highest proportions are in the case of cadmium, lead and manganese, and the most rapid change is for zinc and iron between the MP and LP. These changes are well explained by the increased distance from the inlet of the Matylda waters. Evidently, the small, upper pond was an efficient trap for fine contaminated sediments transported during mining operations from ‘Matylda’. It is also evident from the large amount of sediments filling this pond. Moreover, at present, it still receives sediments containing predominantly organic matter, which is eroded from the contaminated, forested catchment area and is produced in situ due to aquatic plant degradation (Ciszewski et al. 2012).
Despite the decrease in heavy metal concentrations between the small and large pond, there are in any case extreme metal concentrations in the medium and lower pond, exceeding in their centres even 800 mg/kg of Cd, 35,000 mg/kg of Pb and 13,000 mg/kg of Zn. They are similar to the metal content in excavated metal ore and could be related to the accumulation of ore particles (galena and sphalerite), which are abundant in mine-related sediments (Ciszewski et al. 2012). The noticeable influence of the flow current in the middle of the ponds on the metal distribution in sediments seems to be supported by the occurrence of the highest concentrations of all of mine-originated metals along the line linking inlet–outlet boxes in pond dykes. Concentrations decrease in each cross-section toward pond banks with the exception of the left bank in the large pond (LP5, Table 1) where part of the contaminated bottom sediments was moved by bulldozer and restored after the mine closure. Evidently, this measure reduced the contamination of bottom sediments in the large pond as compared to the untouched sediments of the small and medium ponds. Almost all sediment samples from the cascade of ponds are at least several times more contaminated than those from the reference pond. However, in comparison with samples from deeper soil horizons in this area, which are considered unpolluted, it is evident that content of mine-originated metals is higher in each pond than in parent catchment material. The sediments of RP, in particular, are enriched in cadmium and zinc, elements known to be more mobile than lead (Knight et al. 1998; Aleksander-Kwaterczak and Helios-Rybicka 2009; Moreno-Jimenez et al. 2009; Vamerali et al. 2009), which is present there in lower amounts. This suggests a long-term leaching of soils, initially contaminated with the mine waters, which had inundated the upstream valley bottom from the beginning of the mine`s operation (Ciszewski et al. 2012).
In the studied sediments, copper, which is present in generally low concentrations, occurs almost exclusively in the organic–sulphidic fraction (Fig. 2). The highest concentrations of copper occur at the inlet of Matylda waters to the upper pond (SP1, Table 3), and they are probably related to the post-mining activity of asphalt plants in Chrzanów. Nevertheless, the phase speciation of copper in this sample does not depart from that in the others. Its common associations with organic matter are well known and related to sorption and the formation of chelating complexes (Baker 1990; Fytianos and Lourantou 2004; Kierczak et al. 2008). In contrast to copper, zinc and cadmium appear to be more mobile because 60–80 % of these elements, on average, are associated with the reducible fraction. Furthermore, the highest content of iron in the same fraction, about 30–50 %, suggests that most of the cadmium and zinc is associated with iron hydroxides. Only a relatively small portion of Zn, 10–20 %, associated with the exchangeable fraction, is the most mobile and may be easily accessible to aquatic organisms. In contrast to zinc and cadmium, lead is present in the small and large pond mainly in the sulphidic fraction or in the medium pond in the reducible fraction. Overall, the portion of this element in the exchangeable fraction is also small. Manganese is the only element present predominantly in the most mobile, exchangeable fraction. All elements investigated in the sample from the reference pond are associated with the more mobile fractions than those from the cascade of ponds with the exception of iron, present mainly in the biologically inert fraction.
The obtained results of metal fractionation are typical for metal contaminated sediments and indicate the moderate potential mobility of zinc and cadmium. However, zinc may be easily released by ion exchange processes or the dissolution of carbonates and, due to the large sediment contamination; this may result in higher zinc content in pond waters. The largest amount of mine-originated metals in the reducible fraction confirms the findings around the other mines and pollution sources (Siebielec et al. 2006; Aleksander-Kwaterczak and Helios-Rybicka 2009). The metals, associated here rather with iron than manganese oxides, are less available to aquatic organisms but are still labile and may be released upon decomposition of oxides under reduced conditions (Rodriguez et al. 2009). These conditions prevail in the bottom sediments of ponds and are related to the high content of organic matter possibly leading to the release of metals into overlying waters (Table 3). This may be mitigated by water chemistry: principally a pH over neutral, low oxygen and high alkali metal content.
Heavy metals and aquatic plants
Concentrations of heavy metals in studied aquatic plant species ranged between 0.2 and 1.6 mg/kg for Cd, between 2.9 and 370.8 mg/kg for Pb, 1.8 and 8.4 mg/kg for Cu, and 35.1 and 589.5 mg/kg for Zn (Fig. 3). The metals accumulated mainly in the shoots of M. spicatum and the roots of P. australis, while their concentrations were lower in the leaves and steams of P. australis. For instance, in the medium pond, concentrations of Cd, Pb and Zn in the shoots of M. spicatum were higher than in the leaves of P. australis by 3.2, 4.1 and 3 times, respectively. These results are consistent with the frequently observed regularity, indicating that submerged species of macrophytes, especially those belonging to Potamogetonaceae (Demirezen and Aksoy 2004; Fritioff and Greger 2006) accumulated considerably higher concentrations of trace elements in shoots than emergent ones (Baldantoni et al. 2004). These results confirmed that most intensive accumulation of heavy metals was in the roots of P. australis (Baldantoni et al. 2004; Bragato et al. 2006). However, their content is far lower than expected for reeds known to be hyperacumulators. It is known that emergent macrophytes (like P. australis) take up metals from the sediment through the roots (Stoltz and Greger 2002; Weis et al. 2004), whereas only a small portion of metals is transported to the aerial parts (Schierup and Larsen 1981; Baldantoni et al. 2004). High concentrations of metals in the leaves and stems of P. australis are rarely observed (Stoltz and Greger 2002). Concentrations of Cd and Cu in the roots of P. australis and the shoots of M. spicatum were lower, but Pb and Zn fall within the range usually observed for contaminated plants (Deng et al. 2004).
Spatially, concentrations of heavy metals studied in the roots of P. australis change proportionally to those in bottom sediments, i.e. the highest were in the SP and decreased gradually toward the LP. Such a pattern was not observed in the case of the leaves and stems of P. australis. A relationship between heavy metal concentrations in the sediment and plant roots has not always been observed in other studies (Baldantoni et al. 2005; Zhang et al. 2009). However, some investigations have shown a clear relationship between Zn accumulation in aquatic macrophytes and its concentration in sediments (Cardwell et al. 2002). In contrast to these results, positive correlations between concentrations of heavy metals in the sediment and aboveground parts of reed were found by Klink et al. (2009).
Downstream heavy metal changes were also observed for M. spicatum (Fig. 3). The highest concentrations of all metals were found in the shoots of M. spicatum in the SP, while the lowest ones of Cd and Cu were in the LP, Pb in the RP and Zn in the MP. Except for Pb, metal concentrations in shoots did not follow the concentration in water. The high concentrations of heavy metals in the shoots of M. spicatum in the small pond reflected their high concentrations in the water. This was probably related to the strong biosorption properties of M. spicatum (Lesage et al. 2007; Li et al. 2010). Experimental studies showed that the process of Cu and Zn uptake by a plant include rapid sorption on the surface and slow accumulation and translocation in the biomass (Lesage et al. 2007). For instance, the biosorption of Cu(II) by M. spicatum was fast and equilibrium was attained within 20 min (Li et al. 2010). It depends largely on the pH, temperature and concentration of the element (Lesage et al. 2007; Li et al. 2010).
Photosynthetic activity by chlorophyll fluorescence
In situ measurements of Phragmites australis leaves
An analysis of the data obtained for the leaves of P. australis measured along pond banks shows a large internal dispersion of all photosynthetic parameters for individual ponds (see Fig. 4a and b, inserts). Out of the many parameters obtained from the OJIP-test, F V/F M and S M were analysed further. F V/F M average values were the same in SP and RP (0.829 ± 0.003 and 0.829 ± 0.005, respectively), whereas in MP, the analogous ratio was equal to 0.824 ± 0.01, and in LP, the analogous ratio was equal to 0.819 ± 0.011 (see Fig. 4a). Additionally, the values measured in MP and LP apparently belong to two subgroups (see Fig. 4a insert), increasing the dispersion in these data. Average S M (Fig. 4b) was the lowest in SP (64.52 ± 5.54), and in MP, it was significantly lower (68.63 ± 7.78) than in LP and RP (78.26 ± 11.24 and 79.88 ± 7.94, respectively).
Photosynthetic activity of Phragmites australis leaves on Matylda valley pond banks, measures in situ by chlorophyll fluorescence: a average potential photochemical efficiency of photosystem II (F V/F M); b averaged normalised total complementary area above OJIP transient (SM). Inserts particular measured values along measurement transects
Despite a high standard deviation, F V/F M average values for particular ponds are the best, negatively correlated (Pearson corellation test, corellation coefficient = −0.44, significance level = 0.011) to changes in the Cd content in the leaves of reeds (Fig. 4a). Although the average content of heavy metals in the leaves of P. australis in the ponds is relatively small, Cd content shows clearly a clear regular increase down the cascade of ponds (Fig. 3). This trend is opposite to that observed for average Cd content in water and in the sediment of ponds. Nevertheless, the effects of heavy metals, including Cd, on photosynthetic activity are small, which seems to be related to some other environmental factors that affect especially F V/F M at the sites from MP(7) to LP(4).
F V/F M is the parameter that was used for the measurement of changes in photosynthetic activity changes in Phragmites sp. as an effect of different environmental conditions, for example soil type (Zhu et al. 2003), localisation (Meszaros et al. 2003), salinity, alkalinity and N level (Deng et al. 2011) or wastewater treatment (Shelef et al. 2011). The effects of Cd action on P. australis’ photosynthetic apparatus are described in a paper by Pietrini et al. (2003). F V/F M values remained practically stable in plants treated with 50 μM Cd in a hydroponic culture, whereas chlorophyll content and RubisCO activity were significantly affected. Similarly, a prolonged excess of Cu in the soil culture of Phragmites significantly affected the metabolism, growth and chlorophyll content but only had a minute effect on F V/F M (Liu et al. 2009). A high sensitivity of F V/F M was also observed by other investigators but under acute heavy metal stress (Pagliano et al. 2006; Wu et al. 2008; Maleva et al. 2012).
The correlation of F V/F M to Cd content in leaves and its far lower correlation to other heavy metals content probably reflects a decrease in PSII average efficiency caused by a strong inhibition of the PSII photoactivation process by a very low concentration of this element in the chloroplast (Faller et al. 2005). On the other hand, S M is a parameter reflecting multiple-turnover Q A reduction events during F O to F M transient, so it is a relative measure of the functioning of the photosynthetic electron transport chain on the acceptor site of PSII (Strasser et al. 2004). The average values of this parameter are nominatively negative dependent on the content of all four metals in roots, which may be a sign of the disturbance of the whole photosynthetic/bioenergetic machinery by the defence speciation system. S M seems to be a much more effective heavy metal stress-sign than F V/F M, as it is influenced by the physiological status of the whole plant.
Photosynthetic activity in submerged plant shoots
The whole M. spicatum shoots picture was analysed, and the averaged F V/F M was counted (‘whole’ in Fig. 5) for each sample. The separate F V/F M values for each shoot tip in the sample were calculated and then averaged for a sample (‘shoot tips’ in Fig. 5). Additionally, the area of a recorded frame [512(v) × 256(h) pixel] was divided into 10(v) × 20(h) pixel squares, and the minimum and maximum F V/F M values in these squares are presented in Fig. 5 (‘min’ and ‘max’). The values of F V/F M for whole shoots were slightly lower in plants from MP and LP (0.74 for both) than in RP (0.78) and the lowest was in SM, 0.71. Averaged F V/F M for shoot tips in comparison to RP, 0.81 ± 0.01, were only slightly affected in plants from SP, MP and LP, 0.79 ± 0.01, 0.795 ± 0.025 and 0.79 ± 0.01, respectively. The maximum F V/F M registered in squares was the same for RP, MP and LP = 0.82, while it was slightly lower in SP, 0.80. On the other hand, the minimum values of F V/F M registered in squares were significantly lower for plants from the cascade of ponds (SP, 0.58; MP, 0.59; LP, 0.58) than for the reference pond (0.72).
Average potential photochemical efficiency of photosystem II (F V/F M) in Myriophyllum spicatum and Ceratophyllum demersum shoots from ponds of Matylda valley, as analysed by fluorescence imaging. “Whole” average F V/F M for whole apical end of shoots about 15 cm long; “max” and “min” highest and lowest (resp.) values for registered frame; “shoot tips” average F V/F M obtained for appressed young leaves on the shoot tip. Further description in the text
These results could be easily interpreted in the light of the age-dependent response of organs of a related species, Myriophyllum alternifolium, to cadmium and copper, as reported by Delmail et al. (2011a, 2011b). The authors observed that Cd and Cu stresses disturb mainly the structure and function of mature leaves and, in consequence, cause faster ageing and senescence. They considered it a mechanism for limiting the impact of excess metal on young and growing parts of a plant at the expense of mature leaves, which become sinks for metal translocation. In these terms, it seems that the decrease in the ‘max’ value of F V/F M for shoots (only in SP) would mean that complex stress tolerance limits have been exceeded, the lowered ‘min’ value being a sign of the presence of complex environmental stress (in all ponds of the Matylda stream but not in RP), while the average F V/F M value for a whole shoot (‘whole’, see Fig. 5) is a measure of the stress level in the whole plant. Finally, the average value of F V/F M for shoot ends is related to the photosynthetic performance of plants in the environment investigated (as the parameter describing the photosynthesis of the main photosynthetically active part of the plant). The phenomenological description of these parameters needs to be further elucidated, but it seems that a comparison of F V/F M in the young and mature leaves of sensitive submerged plants could be a good test of the quality of the water environment. The results obtained for Myriophyllum coincide with data on heavy metal contents in pond deposits (Table 3) rather than with concentrations in water.
It is of interest that the F V/F M values for C. demersum plants, present in the most contaminated site of SP only, instead of M. spicatum (near site 5, Fig. 1), are practically the same as the parameters of M. spicatum from RP (see Ctph SP in Fig. 5). Considering this in terms described above, it is a sign of the much higher tolerance of C. demersum against stress conditions in SP than that of M. spicatum. This conclusion is additionally confirmed by the continuous presence of Ceratophyllum in SP during the whole vegetation season and by the decline of Myriophyllum in this pond after May. It is well documented in the literature that Myriophyllum has a higher capacity to absorb heavy metal than Ceratophyllum Keskinkan et al. (2007). Additionally, the results of El-Khatib et al. (2011) showed differences between the responses of the antioxidant system of these two water macrophyte species toward heavy metal excess. It seems that these mechanisms may be part of the physiological basis of the differences observed in long-term response in these macrophyte species.
Heavy metals and plankton
The phytoplankton of fishponds was composed of different groups of algae: cyanobacteria, green algae (chlorococcales + desmids), diatoms, chrysophytes, euglenophytes, dinophytes, cryptophytes and xanthophytes. The highest average density of phytoplankton (Fig. 6) was observed in the SP and decreased progressively downstream to the LP (Fig. 1). The density of phytoplankton in the RP was similar to the density in the LP. The composition of predominating groups varied between the ponds analysed. Diatoms dominated in the SP, MP and LP ponds, but their percentage in the total density of phytoplankton decreased from SP to LP ponds (Fig. 7). Simultaneously, the share of green algae and cyanobacteria in total density increased. The density of others groups was similar in the cascade of ponds. The RP was dominated by chrysophytes, while diatoms and green algae were less abundant when compared to the cascade of ponds. In this pond, we did not observe any cyanobacteria species.
The nutrients (P and N) are crucial for the regulating of the phytoplankton composition. However, in the studied ponds, we did not observe deficiency of phosphorus. The values of concentrations were similar in the all ponds, so that factor did not affect phytoplankton density and the structure (dominating groups). It seems that nitrogen was the more important factor influenced on phytoplankton development. Nitrogen limitation appears often to eutrophic waters (Harris 1994), and the investigated ponds are eutrophic. Although ammonia nitrogen did not show differences in the ponds, so the only one factor affected the phytoplankton was nitrate nitrogen. In fact, in the small pond where the highest concentration of nitrate nitrogen was observed, the highest density of phytoplankton with dominating diatoms was noted. In addition, there are many other studies that showed that high develop of diatoms is related to high concentration of nitrate nitrogen (e.g. Patrick 1977, Wilk-Woźniak and Kosiński 2001). In the medium, large and reference ponds the nitrate nitrogen concentration decreased, and the density of phytoplankton also decreased.
There are mostly negative opinions about the effects of heavy metals on planktonic organisms (e.g. Balistrieri et al. 2007). However, some observations showed adaptation to prolonged heavy metal contamination (Pawlik-Skowronska 2002a; Ligeza and Wilk-Wozniak 2011; Wilk-Wozniak et al. 2011). In the investigated fishponds, there were many algal taxa, which are considered to be taxa characteristic of waters contaminated with heavy metals, which were numerous or even dominated. There were species belonging to Navicula, Nitzschia and Synedra (diatoms), and Desmodesmus (green algae). In the reference pond, a domination of species which are neither resistant nor tolerant to heavy metals (Dinobryon divergens—chrysophytes) was observed. It seems that algae populations can quite easily adjust to long-lasting contamination. However, there are other factors that may explain the existence of microorganisms inhabiting contaminated water. One is the presence of high concentrations of Ca and Mg cations. It is known that these cations can limit the penetration of high amounts of Zn and Pb into cells (Pawlik-Skowronska 2002b). It is also known that algae can respond to metal combinations, e.g. the Cu–Ni combination results in synergistic interactions, in contrast to the antagonism of Cu–Fe and Ni–Fe. This means that iron may mask the effect of Cu and Ni. There are also some observations that the toxicity of Cu increases in the presence of Fe, while the toxicity of Cd increases in the presence of Pb and decreases in the presence of Ca, Fe and Zn (Whitton and Shehata 1982).
The zooplankton of the fishponds was composed of only three groups: rotifers, copepods and cladocerans. The highest average density of zooplankton was observed in the MP while, the lowest density was in the RP. The density of zooplankton in the SP was similar to that in the LP (Fig. 6). Rotifers dominated in every fishpond investigated (Fig. 8). In the RP, their contribution to the total density of zooplankton was <90 % and their content in the cascade of ponds was always >90 %. In the case of copepods and cladocerans, their density in ponds constituted from 0 to 13 % of total zooplankton density.
Rotifers dominated in all ponds, but the domination of rotifers is known and common in freshwater ecosystems (Sarma et al. 2007a). However, this group is also known to be the least sensitive to pollution, e.g. with heavy metals (Jak et al. 1996). The composition of rotifer species also varies in aquatic ecosystems with high patchiness of aquatic vegetation (Bielańska-Grajner and Gładysz 2010). It is possible that individuals that live in fishponds are clones resistant to the negative effects of heavy metals. Sarma et al. (2007a) suggested that resistance or sensitivity to heavy metals, e.g. the concentration of Zn in particular rotifer species, might have a positive or negative influence. Other groups such as Cladocera and Copepoda had a smaller share in the total density of zooplankton. It is our opinion that this phenomenon is a result of long-term sediment contamination by heavy metals, since both groups are sensitive to heavy metals, especially Cladocera Sarma et al. (2007b). The highest density of zooplankton in the MP showed small impact of heavy metals dissolved in the water on zooplankton. This could reflect the adaptation of zooplankton communities to long-lasting contamination.
Effects of sediment contamination on aquatic organisms
It is well recognised that mine-originating sediments act as secondary contributors of metal contamination to the aquatic environment. Metals contained in sediments are known to affect mostly benthic organisms depending on metal speciation, sediment-water partitioning, organisms physiology and feeding behavior (Aleksander-Kwaterczak et al. 2009), while the toxicity of sediments for the other aquatic organisms is controlled to a larger extent by the physiochemical parameters of waters and reservoirs (Suchanek et al. 2008). In the present studies, the recent history of water contamination in ponds is not known in detail, although the main stream, which supplies the cascade of ponds, is known to be moderately to weakly polluted and in its middle reach metal contamination is similar to that of pond water (Aleksander-Kwaterczak and Ciszewski 2012; Ciszewski et al. 2012). Moreover, we may reasonably suppose that, based on long-term studies in other mining areas (Rainbow et al. 2011), metal content in water could be higher soon after the closure of a mine. This suggests that for the 40 years subsequent to the end of mining the pond waters have been permanently contaminated with heavy metals albeit to a moderate degree as a result of the remobilisation of metals from sediments. These processes alternate spatially as indicated by the variable metal concentrations down the cascade of ponds and without regular changes in time probably as a result of complicated processes of sorption/desorption and precipitation as well as the variable inflow of more and less contaminated main and side stream waters. Nevertheless, metal mobilisation, limited in ponds due to widespread anoxic conditions, high alkali metal content and pH over neutral, explains the metal concentrations in water and plants, which are not low proportionally to sediment contamination in ponds. This eliminates the acute effects of sediment contamination on aquatic organisms, which were not observed here. Instead, the aquatic plants and plankton investigated seem to be adjusted to the prolonged contamination.
Photosynthetic activity in Phragmites communis is practically unaffected in the plants of the Matylda ponds. M. spicatum showed, however, noticeable differences in F V/F M distribution between developing, mature and senescing leaves, which was dependent on sediment contamination (which seems to correspond to average water contamination). This phenomenon could be interpreted as the effect of the cumulation of heavy metal-induced damages in plant tissues during the vegetation season. Moreover, in the extreme conditions of site 5 in SP, these metabolic disturbances can lead to the displacement of the more invasive but less contaminant-resistant Myriophyllum by less sensitive Ceratophyllum species.
Many studies have shown a negative response of planktonic algae (low density, low diversity, presence of single species, etc.) to the contamination of waters with heavy metals (Wolowski et al. 2008). However, our investigations did not show such strong effects of contamination on phytoplankton. The density of phytoplankton in all ponds was quite high. Nevertheless, the presence of species (diatoms, green algae) resistant to high concentrations of heavy metals might suggest (Pawlik-Skowronska 2002a), that planktonic communities have adapted to the chronic and persistent heavy metal concentration in sediments.
Among zooplanktons, Rotifera was the most abundant group. This group is known to be the least sensitive to pollution (Jak et al. 1996). It is possible that rotiferas that live in fishponds contaminated with heavy metals have been adapted to withstand the negative effects of heavy metals. Groups such as Cladocera and Copepoda, which are more sensitive to heavy metals (Sarma et al. 2007b), were less abundant. The high density and diversity of zooplankton in the fishponds affected by heavy metals, proved that as with phytoplankton, plankton fauna adapt to long-lasting contamination.
Conclusions
Peak mining activity in the Matylda mine, which lasted over 20 years and finished in the early 1970’s, resulted in the supply of mine-originating sediments to fish ponds which are currently used for recreational angling. The heavy metal-contaminated sediments have been trapped in ponds and, together with the mine water-logged soils of the valley bottom, have become a long-term source of heavy metals. Widespread anoxic conditions in bottom sediments, the high alkali metal content and the neutral to high pH values control the release of metals to overlying waters. In effect, heavy metal concentrations in waters, however persistently high, are low when compared to most former mining areas. Moreover, at present the ponds are supplied with well oxygenated waters, which, apart from the small pond filled to the largest extent with strongly contaminated sediments, are of good quality. Both present and reconstructed changes in the water and sediment quality over the Matylda catchment (Ciszewski et al. 2012) suggest that, for about the last 40 years, aquatic organisms have been suffering more from persistent than from strong contamination with heavy metals, and there are not such no acute effects on aquatic organisms as could be expected from extremely high sediment contamination.
Concentrations of Cd and Cu in the macrophytes studied were low, but those of Pb and Zn were more characteristic of contaminated plants. The concentrations of heavy metals in the roots of P. australis were to some extent proportional to those in the sediments of ponds. The stronger biosorption properties of M. spicatum, are probably related to the high concentrations of heavy metals in the shoots of M. spicatum, and may reflect the raised concentrations, which periodically occur in the water. Because macrophytes growing in the Matylda ponds have adapted to elevated concentrations of heavy metals over the long period of contamination, changes in photosynthesis physiology are visible only as differences in the activity distribution in leaves of different ages. On the other hand, it seems that the highest level of contamination in SP has led to the disappearance of M. spicatum and its reservoir and its displacement by Ceratophyllum.
Heavy metal concentrations in fishponds did not affect the density of phyto- and zooplankton, but it seems that they had an impact on their composition. This is suggested by the domination of diatoms and green algae in the phytoplankton of the fishponds with sediments, which are most contaminated by heavy metals. In the reference pond, the dominant group was Chrysophytes.
Among zooplankton Rotifers were the most abundant but in the reference pond their contribution was <90 %. The dominance of less-sensitive species in plankton communities may suggest that long-lasting contamination of sediments by heavy metals had little effect on planktonic organisms; because their life cycle is fast, they can adopt to environmental conditions quickly.
Although these studies have suggested low metal concentrations in fish, further research is required in order to provide evidence on the safety of fish caught by anglers in these ponds.
References
Aleksander-Kwaterczak, U., & Helios-Rybicka, E. (2009). Contaminated sediments as a potential source of Zn, Pb, and Cd for a river system in the historical metalliferous ore mining and smelting industry area of South Poland. Journal of Soils and Sediments, 9(1), 13–22.
Aleksander-Kwaterczak, U., Mazurek, M., & Wardas, M. (2009). The use of physicochemical and biological quality elements for the determination of aquatic environment quality as shown by the example of watercourses in the surroundings of the Żelazny Most flotation tailings pond. Polish Journal of Environmental Studies, 18(2), 56–63.
Aleksander-Kwaterczak, U., & Ciszewski, D. (2012). Groundwater hydrochemistry and soil pollution in a catchment affected by an abandoned lead-zinc mine: Functioning of a diffuse pollution source. Environmental Earth Science, 65, 1179–1189.
Alvarez, E., Fernandez Marcos, M. L., Vaamonde, C., & Fernandez-Sanjurio, M. J. (2003). Heavy metals in the dump of an abandoned mine in Galicia (NW Spain) and in the spontaneously occurring vegetation. The Science of the Total Environment, 313, 185–197.
Baldantoni, D., Alfani, A., Di Tommasi, P., Bartoli, G., & De Santo, A. V. (2004). Assessment of macro and microelement accumulation capability of two aquatic plants. Environmental Pollution, 130, 149–156.
Baldantoni, D., Maisto, G., Bartoli, G., & Alfani, A. (2005). Analyses of three native aquatic plant species to assess spatial gradients of lake trace element contamination. Aquatic Botany, 83, 48–60.
Baker, D. E. (1990). Copper. In B. J. Alloway (Ed.), Heavy metals in soils (pp. 151–176). Glasgow: Blackie Academic & Professional.
Balistrieri, L. S., Seal, R. R., II, Piatak, N. N., & Paul, B. (2007). Assessing the concentration, speciation, and toxicity of dissolved metals during mixing of acid-mine drainage and ambient river water downstream of the Elizabeth Copper Mine. Applied Geochemistry, 22, 930–952.
Bielańska-Grajner, I., & Gładysz, A. (2010). Plankotnic rotifers in mining lakes in the Silesian Upland: Relationships to environmental parameters. Limnologica, 40, 67–72.
Bragato, C., Brix, H., & Malagoli, M. (2006). Accumulation of nutrients and heavy metals in Phragmites australis (Cav.) Trin. ex Steudel and Bolboschoenus maritimus (L.) Palla in a constructed wetland of the Venice lagoon watershed. Environmental Pollution, 144, 967–975.
Byrne, P., Reid, I., & Wood, P. J. (2010). Sediment geochemistry of streams draining abandoned lead/zinc mines in central Wales: The Afon Twymyn. Journal of Soils and Sediments, 10, 683–697.
Cardwell, A. J., Hawker, D. W., & Greenway, M. (2002). Metal accumulation in aquatic macrophytes from southeast Queensland, Australia. Chemosphere, 48, 653–663.
Cattaneo, A., Couillard, Y., Wunsam, S., & Courcelles, M. (2004). Diatom taxonomic and morphological changes as indicators of metal pollution and recovery in Lac Dufault (Quebec, Canada). Journal of Paleolimnology, 32, 163–175.
Ciszewski, D. (2004). Pollution of the Mała Panew sediments with heavy metals: Part I. Effect of changes in river bed morphology. Pollution of Journal Environmental Studies, 13, 589–595.
Ciszewski, D., Kubsik, U., & Aleksander-Kwaterczak, U. (2012). Long-term dispersal of heavy metals in a catchment affected by historical lead and zinc mining. Journal of Soils and Sediments, 12, 1445–1462.
Crowder, A. (1991). Acidification, metals and macrophytes. Environmental Pollution, 71, 171–203.
Delmail, D., Labrousse, P., Hourdin, P., Larcher, L., Moesch, C., & Botineau, M. (2011a). Physiological, anatomical and phenotypical effects of a cadmium stress in different-aged chlorophyllian organs of Myriophyllum alterniflorum DC (Haloragaceae). Environmental and Experimental Botany, 72, 174–181.
Delmail, D., Labrousse, P., Hourdin, P., Larcher, L., Moesch, C., & Botineau, M. (2011b). Differential responses of Myriophyllum alterniflorum DC (Haloragaceae) organs to copper: Physiological and developmental approaches. Hydrobiologia, 664, 95–105.
Demirezen, D., & Aksoy, A. (2004). Accumulation of heavy metals in Typha angustifolia (L.) and Potamogeton pectinatus (L.) living in Sultan Marsh (Kayseri, Turkey). Chemosphere, 56, 685–696.
Deng, H., Ye, Z., & Wong, M. (2004). Accumulation of lead, zinc, copper and cadmium by 12 wetland plant species thriving in metal-contaminated sites in China. Environmental Pollution, 132, 29–40.
Deng, C., Zhang, G., & Pan, X. (2011). Photosynthetic responses in reed (Phragmites australis (CAV.) TRIN. ex Steud.) seedlings induced by different salinity–alkalinity and nitrogen levels. Journal of Agricultural Science Technology, 13, 687–699.
Dogan, M., & Saygideger, S. (2004). Influence of pH on lead accumulation, chlorophyll and nitrogen content of Polygonum salicifolium Brouss. ex Willd. Fresenius Environmental Bulletin, 13, 777–782.
El-Khatib, A. A., Hegazy, A. K., & Abo-El-Kassem, A. (2011). Cadmium induced response of protein profile and antioxidant enzymes in aquatic macrophytes Myriophyllum spicatum and Ceratophyllum demersum. Journal of Environmental Studies, 7, 17–23.
Escarre, J., Lefebvre, C., Raboyeau, S., Dossantos, A., Gruber, W., Marel, J. C. C., et al. (2011). Heavy metal concentration survey in soils and plants of the Les Malines mining district (southern France): Implications for soil restoration. Water, Air, and Soil Pollution, 216, 485–504.
Faller, P., Kienzler, K., & Krieger-Liszkay, A. (2005). Mechanism of Cd2+ toxicity: Cd2+ inhibits photoactivation of Photosystem II by competitive binding to the essential Ca2+ site. Biochimica et Biophysica Acta, 1706, 158–164.
Franzin, W. G., & McFarlane, G. A. (1980). An analysis of the aquatic macrophyte Myriophyllum exalbescens as an indicator of metal contamination of aquatic ecosystems near base metal smelter. Bulletin Environmental Contaminants Toxicology, 24, 597–605.
Fritioff, A., & Greger, M. (2006). Uptake and distribution of Zn, Cu, Cd, and Pb in an aquatic plant Potamogeton natans. Chemosphere, 63, 220–227.
Fytianos, K., & Lourantou, A. (2004). Speciation of elements in sediment samples collected at lakes Volvi and Koronia, N. Greece Environmental International, 30, 11–17.
Harris G.P. (1994). Nutrients loadings and algal blooms in Australia waters—A discussion paper. Land and Water Resources Research and Development Corporation, Occasional Paper 12, Canberra.
Hutchinson, S. M., & Rothwell, J. J. (2008). Mobilisation of sediment-associated metals from historical Pb working sites on the River Sheaf, Sheffield. UK. Environment Pollution, 155, 61–71.
Jackson, T. A., & Bistricki, T. (1995). Selective scanvenging of copper, zinc, lead and arsenic by iron and manganese oxyhydroxide coatings on plankton in lakes polluted with mine and smelter wastes: Results of energy dispersive X-ray micro-analysis. Journal of Geochemical Exploration, 52, 97–125.
Jackson, L. J., Kalff, J., & Rasmussen, J. J. (1996). Sediment pH and redox potential affect the bioavailability of Al, Cu, Fe, Mn and Zn to rooted aquatic macrophytes. Canadian Journal of Fisheries and Aquatic Sciences, 50, 143–148.
Jak, R. G., Maas, J. I., & Scholten, M. C. T. (1996). Evaluation of laboratory derived toxic effect concentrations of a mixture of metals by testing freshwater plankton communities in enclosures. Water Research, 30, 1215–1227.
Kelly, M. S., & Pinder, J. E. (1996). Foliar uptake of 137Cs from the water column by aquatic macrophytes. Journal of Environmental Radioactivity, 30, 271–280.
Keskinkan, O., Goksu, M. Z. L., Yuceer, A., & Basibuyuk, M. (2007). Comparison of the adsorption capabilities of Myriophyllum spicatum and Ceratophyllum demersum for zinc. Copper and Leadership Engineering Life Science, 7, 192–196.
Kierczak, J., Neel, C., Aleksander-Kwaterczak, U., Helios-Rybicka, E., Bril, H., & Puziewicz, J. (2008). Solid speciation and mobility of potentially toxic elements from natural and contaminated soils: A combined approach. Chemosphere, 73(5), 776–784.
Klink, A., Krawczyk, J., & Wislocka, M. (2009). The content of heavy metals in leaves of Phragmites australis (Cav.) Trin.ex Steud. and bottom sediments from lakes of Pojezierze Leszczyńskie. Ochrona Środowiska i Zasobów Naturalnych, 39, 60–66.
Knight, B. P., Chaudri, A. M., McGrath, S. P., & Giller, K. E. (1998). Determination of chemical availability of cadmium and zinc in soils using inert soil moisture samplers. Environmental Pollution, 99, 293–298.
Lesage, E., Mundia, C., Rousseau, D. P. L., Van de Moortel, A. M. K., Du Laing, G., Meers, E., et al. (2007). Sorption of Co, Cu, Ni and Zn from industrial effluents by the submerged aquatic macrophyte Myriophyllum spicatum L. Ecological Engineering, 30, 320–325.
Lewander, M., Szarek, E., & Greger, M. (1996). Macrophytes as indicators of bioavailable Cd, Pb and Zn flow in the river Przemsza, Katowice Region. Applied Geochemistry, 11, 169–173.
Li, G., Xue, P., Yan, C., & Li, Q. (2010). Copper biosorption by Myriophyllum spicatum: Effects of temperature and pH. Korean Journal of Chemistry Engineering, 27(4), 1239–1245.
Lichtenthaler, H. K., Buschmann, C., & Knapp, M. (2005). How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica, 43, 379–393.
Ligeza, S., & Wilk-Wozniak, E. (2011). The occurrence of Euglena pascheri and Lepocinclis ovum bloom in an oxbow Lake in southern Poland under extreme environmental conditions. Ecological Indicators, 11, 925–929.
Lim, H. S., Lee, J. S., Chon, H. T., & Sager, M. (2008). Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au–Ag mine in Korea. Journal of Geochemical Exploration, 96, 223–230.
Liu, X., Shen, Y., Lou, L., Ding, C., & Cai, Q. (2009). Copper tolerance of the biomass crops Elephant grass (Pennisetum purpureum Schumach), Vetiver grass (Vetiveria zizanioides) and the upland reed (Phragmites australis) in soil culture. Biotechnology Advances, 27, 633–640.
Lukin, A., Dauvalter, V., Kashulin, N., Yakovlev, V., Sharov, A., & Vandysh, O. (2003). Assessment of copper–nickel industry impact on subarctic lake ecosystem. The Science of the Total Environment, 306, 73–83.
Lund, J. W. G., Kipling, G., & Le Cren, E. D. (1958). The inverted microscope method of estimating algae numbers and the statistical basis of estimation by counting. Hydrobiologia, 11, 143–170.
Maleva, M. G., Nekrasova, G. F., Borisova, G. G., Chukina, N. V., & Ushakova, O. S. (2012). Effect of heavy metals on photosynthetic apparatus and antioxidant status of elodea. Russian Journal of Plant Physiology, 59, 190–197.
Markich, S. J., King, A. R., & Wilson, S. P. (2006). Non-effect of water hardness on the accumulation and toxicity of copper in a freshwater macrophyte (Ceratophyllum demersum): How useful are hardness-modified copper guidelines for protecting freshwater biota? Chemosphere, 65, 1791–1800.
Marques, M. J., Martinez-Conde, E., Rovira, J. V., & Ordonez, S. (2001). Heavy metals pollution of aquatic ecosystems in the vicinity of a recently closed underground lead–zinc mine (Basque Country, Spain). Environmental of Geology, 40, 1125–1137.
Mazurkiewicz-Boroń, G. (2002). Factors of eutrophication processes in sub-mountain dam reservoirs. Supplementa ad Acta Hydrobiologica, 2, 1–68.
Meszaros, I., Veres, S., Dinka, M., & Lakatos, G. (2003). Variations in leaf pigment content and photosynthetic activity of Phragmites australis in healthy and die-back reed stands of Lake Fertõ/Neusiedlersee. Hydrobiologia, 506, 681–686.
Mighanetara, K., Braungardt, C. B., Rieuwerts, J. S., & Azizi, F. (2009). Contaminant fluxes from point and diffuse sources from abandoned mines in the river Tamar catchment, UK. Journal of Geochemicla Exploration, 100, 116–124.
Mishra, V. K., Upadhyaya, A. R., Pandey, S. K., & Tripathi, B. D. (2008). Heavy metal pollution induced due to coal mining effluent on surrounding aquatic ecosystem and its management through naturally occurring aquatic macrophytes. Bioresource Technology, 99, 930–936.
Moreno-Jimenez, E., Penalosa, J. M., Manzano, R., Carpena-Ruiz, R. O., Gamarra, R., & Esteban, E. (2009). Heavy metals distribution in soils surrounding an abandoned mine in NW Madrid (Spain) and their transference to wild flora. Journal of Hazardous Materials, 162, 854–859.
Nikolaidis, C., Zafiriadis, I., Mathioudakis, V., & Constantinidis, T. (2010). Heavy metal pollution associated with an abandoned lead–zinc mine in the Kirki region. NE Greece. Bull Environ Contam Toxicol, 85(3), 307–312.
Pagliano, C., Raviolo, M., Dalla Vecchia, F., Gabbrielli, R., Gonnelli, C., Rascio, N., et al. (2006). Evidence for PSII donor-side damage and photoinhibition induced by cadmium treatment on rice (Oryza sativa L.). Journal of Photochemistry and Photobiology Biology, 84, 70–78.
Pasieczna, A. (Ed.). (2011). Detailed geochemical map of Upper Silesia, Chrzanów sheet 1:25000. Warszawa: Państwowy Instytut Geologiczny.
Patrick, R. (1977). Ecology of freshwater diatoms and diatom communities. In D. Werner (Ed.), The biology of diatoms, Bot. Monogr. 13 (pp. 284–332). Oxford: Blackwell.
Pawlik-Skowronska, B. (2002a). Tajemnice odporności glonów i sinic na toksyczne metale ciężkie. Kosmos, 51, 175–184.
Pawlik-Skowronska, B. (2002b). Correlations between toxic Pb effects and production of Pb-induced thiol peptides in the microalga Stichococcus bacillaris. Environmental Pollution, 119, 119–127.
Pietrini, F., Iannelli, M. A., Pasqualini, S., & Massacci, A. (2003). Interaction of cadmium with glutathione and photosynthesis in developing leaves and chloroplasts of Phragmites australis (Cav.) Trin. ex Steudel. Plant Physiology, 133, 829–837.
Pociecha, A., Higgins, T., & McCarthy, T. K. (2010). A preliminary study on the plankton assemblages of Lough Derg (Ireland) during a winter–spring season. Ocean. Hydrob. Stud, 39, 145–154.
Rauret, G., Lopez-Sanchez, J. F., Sahuquillo, A., Rubio, R., Davidson, C., Ure, A., et al. (1999). Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. Journal of Environmental Monitoring, 1, 57–61.
Rodriguez, L., Ruiz, E., Alonso-Azcarate, J., & Rincon, J. (2009). Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. Journal of Environmental Management, 90, 1106–1116.
Salonen, V. P., Tuovinen, N., & Valpola, S. (2006). History of mine drainage impact on Lake Orijärvi algal communities, SW Finland. Journal of Paleolimnology, 35, 289–303.
Sánchez, J., Marino, N., Vaquero, M. C., Ansorena, J., & Legórburu, I. (1998). Metal pollution by old lead-zinc mines in Urumea River valley (Basque Country, Spain). Soil, biota and sediment. Water. Air and Soil Pollution, 107, 303–319.
Sarma, S. S. S., Azuara-Garcia, R., & Nandini, S. (2007a). Combined effects of zinc and algal food on the competition between planktonie rotifers, Anuraeopsis fissa and Brachionus Rubens (Rotifera). Aquatic Ecology, 41, 631–638.
Sarma, S. S. S., Peredo-Alvarez, V. M., & Nandini, S. (2007b). Comparative study of the sensitivities of neonates and adults of selected cladoceran (Cladocera: Crustacea) species to acute toxicity stress. Journal of Environmental Science Health A, 42, 1449–1452.
Sarvari E. (2005). Effects of heavy metals on chlorophyll–protein complexes in higher plants: causes and consequences (pp. 865–888). In M. Pessarakli (Ed.) Handbook of photosynthesis. Boca Raton: Taylor and Francis
Schierup, H. H., & Larsen, V. J. (1981). Macrophyte cycling of zinc, copper, lead and cadmium in the littoral zone of a polluted and a non-polluted lake. I. Availability, uptake and translocation of heavy metals in Phragmites australis (Cav.). Trin. Aquatic Botany, 11, 197–210.
Shelef, O., Golan-Goldhirsh, A., Gendler, T., & Rachmilevitch, S. (2011). Physiological parameters of plants as indicators of water quality in a constructed wetland. Environmental Science and Pollution Research, 18, 1234–1242.
Shinmachi, F., Kumanda, Y., Noguchi, A., & Hasegawa, I. (2003). Translocation and accumulation of cadmium in cadmium-tolerant Polygonum thunbergii. Soil Science and Plant Nutrition, 49(3), 355–361.
Shubert, E., Rusu, A.-M., Bartok, K., & Moncrieff, C. B. (2001). Distribution and abundance of edaphic algae adapted to highly acidic, metal rich soils. Nova Hedwigia, 123, 411–425.
Siebielec, G., Stuczyński, T., & Korzeniowska-Pucułek, R. (2006). Metal bioavailability in long-term contaminated tarnowskie gory soils. Polish Journal of Environmental Studies, 15(1), 121–129.
Srivastava, J., Gupta, A., & Chandra, H. (2008). Managing water quality with aquatic macrophytes. Reviews in Environmental Science and Biotechnology, 7, 255–266.
Stoltz, E., & Greger, M. (2002). Accumulation properties of As, Cd, Cu, Pb and Zn by four wetland plant species growing on submerged mine tailings. Environmental and Experimental Botany, 47, 271–280.
Strasser, R. J., Tsimilli-Michael, M., & Srivastava, A. (2004). Analysis of the chlorophyll a fluorescence transient. In G. C. Papageorgiou & Govindjee (Eds.), Chlorophyll fluorescence: A signature of photosynthesis (pp. 321–362). Dordrecht: Kluwer.
Suchanek, T. H., Eagles-Smith, C. A., & Harner, E. J. (2008). Is Clear Lake methylmercury distribution decoupled from bulk mercury loading? Ecology Application, 18, 107–127.
Weis, J. S., Glover, T., & Weis, P. (2004). Interactions ofmetals affect their distribution in tissues of Phragmites australis. Environmental Pollution, 131, 409–415.
Welsh, R. P. H., & Denny, P. (1980). The uptake of lead and copper by submerged aquatic macrophytes in two English lakes. Journal of Ecology, 68, 443–455.
Whitton, B. A., & Shehata, H. A. (1982). Influence of cobalt, nickel, copper and cadmium on the blue-green alga Anacystis nidulans. Environmental Pollution Series A, Ecological and Biological, 27(4), 275–281.
Wilk-Wozniak E. (2009). Zmiany populacyjne w zbiorowiskach glonów planktonowych oraz ich strategie życiowe w warunkach ekosystemów wodnych sztucznie zmienionych (Changes in phytoplankton communities and the life strategies of planktonic algae in artificially changed aquatic ecosystems). Studia Naturae 55, IOP PAN, Kraków. (in Polish).
Wilk-Woźniak, E., & Kosiński, M. (2001). Effect of allochthonous and autochthonous factors on phytoplankton biomass in a submontane dam reservoir (S Poland). Biologia, 56(4), 345–354.
Wilk-Wozniak, E., Pociecha, A., Ciszewski, D., Aleksander-Kwaterczak, U., & Walusiak, E. (2011). Phyto-and zooplankton in fishponds contaminated with heavy metals runoff from a lead–zinc mine. Oceanological and Hydrobiological Studies, 40, 77–85.
Wilson, B., & Pyatt, F. B. (2007). Heavy metal dispersion, persistence and bioaccumulation around an ancient copper mine situated in Anglesey. UK. Ecotoxicology and Environmental Safety, 66, 224–231.
Wolowski, K., Turnau, K., & Henriques, F. S. (2008). The algal flora of an extremely acidic, metal-rich drainage pond of Săo Domingos pyrite mine (Portugal). Cryptogamie Algol, 29, 313–324.
Wu, X., Hong, F., Liu, C., Su, M., Zheng, L., Gao, F., et al. (2008). Effects of Pb2+ on energy distribution and photochemical activity of spinach chloroplast. Spectrochimica Acta Part A, 69, 738–742.
Vamerali, T., Bandiera, M., Coletto, L., Zanetti, F., Dickinson, N. M., & Mosca, G. (2009). Phytoremediation trials on metal- and arsenic-contaminated pyrite wastes(Torviscosa, Italy). Environmental Pollution, 157, 887–894.
Younger, P. L. (1997). Longevity of mine water pollution: A basis for decision-making. The Science of the Total Environment, 194/195, 457–466.
Zhang, M., Cuib, L., Sheng, L., & Wang, Y. (2009). Distribution and enrichment of heavy metals among sediments, water body and plants in Hengshuihu Wetland of Northern China. Ecological Engineering, 35, 563–569.
Zhu, X. Y., Wang, Z. M., & Zhang, C. L. (2003). Composition and characteristic differences in photosynthetic membranes of two ecotypes of reed (Phragmites communis L) from different habitats. Photosynthetica, 41, 97–104.
PN-EN ISO 17294–1:2007. http://www.khgi.agh.edu.pl/i/pdf/ibok-ab1050/ab1050.pdf
Acknowledgements
The work was funded by the Ministry of Science and Higher Education grant no. N N305 232 735 and University of Science and Technology in Krakow grant no. 11.11.140.199.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Ciszewski, D., Aleksander-Kwaterczak, U., Pociecha, A. et al. Small effects of a large sediment contamination with heavy metals on aquatic organisms in the vicinity of an abandoned lead and zinc mine. Environ Monit Assess 185, 9825–9842 (2013). https://doi.org/10.1007/s10661-013-3295-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3295-z