Abstract
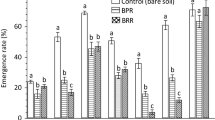
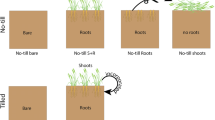
Phenolic allelochemicals have been found in both natural and managed ecosystems, where they cause numerous ecological and economic problems. Whether these problems can be mediated by some other specific phenolic acid components is unknown. In this study, we identified phenolic acids and their concentrations in plow layer soil, rhizosphere soil and decomposing strawberry (Fragaria ananassa Duch.‘Benihoppe’.) plants susceptible to strawberry anthracnose crown rot. We also assessed the effects of exogenously added phenolic acids at varying concentrations on Colletotrichum gloeosporioides, the pathogen causing strawberry anthracnose crown rot, conidial germination and colony growth. Finally, we verified the occurrence of strawberry anthracnose crown rot and the changes in root structure in response to phenolic acids. Ten phenolic acids were identified in soil samples. The concentrations of p-coumaric acid (PA) and ferulic acid (FA) were higher than other phenolic acids. Relatively high concentrations of PA and FA could increase the occurrence of strawberry seedling anthracnose crown rot. However, when the concentrations of PA and FA were higher than respectively certain critical concentration, they could reduce the degree of the disease. Meanwhile, high concentration of FA seriously inhibited the growth of root. The trans-cinnamic acid (TA) content could be regulated to control the occurrence of strawberry anthracnose crown rot without affecting root growth. Overall, diverse phenolic acids in plow soil had different influence on strawberry anthracnose crown rot. The effects of phenolic acids were concentration-dependent and C. gloeosporioides was more sensitive to phenolic acids concentration than root.






Similar content being viewed by others
References
Asaduzzaman, M., Kobayashi, Y., Isogami, K., Tokura, M., Tokumasa, K., & Asao, T. (2012). Growth and yield recovery in strawberry plants under autotoxicity through electrodegradation. European Journal of Horticultural Science, 77(2), 58–67.
Asao, T., Kitazawa, H., Ban, T., & Habibur Rahman Pramanik, M. (2008). Electrodegradation of root exudates to mitigate autotoxicity in hydroponically grown strawberry (Fragaria × ananassa Duch.) plants. Hort Science, 43(7), 2034–2038.
Atanasova-Penichon, V., Pons, S., Pinson-Gadais, L., Picot, A., Marchegay, G., Bonnin-Verdal, M.-N., et al. (2012). Chlorogenic acid and maize ear rot resistance: a dynamic study investigating Fusarium graminearum development, deoxynivalenol production, and phenolic acid accumulation. Molecular Plant-Microbe Interactions, 25(12), 1605–1616. doi:10.1094/mpmi-06-12-0153-r.
Baker, K. F., & Cook, R. J. (1974). Biological control of plant pathogens (biological control of plant pathogens).
Berendsen, R. L., Pieterse, C. M. J., & Bakker, P. A. H. M. (2012). The rhizosphere microbiome and plant health. Trends in Plant Science, 17(8), 478–486. doi:10.1016/j.tplants.2012.04.001.
Blum, U., & Shafer, S. R. (1988). Microbial-populations and phenolic-acids in soil. Soil Biology & Biochemistry, 20(6), 793–800. doi:10.1016/0038-0717(88)90084-3.
Bonanomi, G., Antignani, V., Capodilupo, M., & Scala, F. (2010). Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases. Soil Biology and Biochemistry, 42(2), 136–144. doi:10.1016/j.soilbio.2009.10.012.
Boutigny, A.-L., Atanasova-Penichon, V., Benet, M., Barreau, C., & Richard-Forget, F. (2010). Natural phenolic acids from wheat bran inhibit Fusarium culmorum trichothecene biosynthesis in vitro by repressing Tri gene expression. European Journal of Plant Pathology, 127(2), 275–286. doi:10.1007/s10658-010-9592-2.
Cao, K. Q., & Wang, S. T. (2007). Autotoxicity and soil sickness of strawberry (Fragaria x ananassa). Allelopathy Journal, 20(1), 103–113.
Chen, L., Yang, X., Raza, W., Li, J., Liu, Y., Qiu, M., et al. (2011a). Trichoderma harzianum SQR-T037 rapidly degrades allelochemicals in rhizospheres of continuously cropped cucumbers. Applied Microbiology and Biotechnology, 89(5), 1653–1663. doi:10.1007/s00253-010-2948-x.
Chen, S., Zhou, B., Lin, S., Li, X., & Ye, X. (2011b). Accumulation of cinnamic acid and vanillin in eggplant root exudates and the relationship with continuous cropping obstacle. African Journal of Biotechnology, 10(14), 2659–2665.
Chou, C. H. (1999). Roles of allelopathy in plant biodiversity and sustainable agriculture. Critical Reviews in Plant Sciences, 18(5), 609–636. doi:10.1016/s0735-2689(99)00393-7.
Dalton, B.R., Weed, S.B. & Blum, U. (1987). Plant phenolic acids in soils: a comparison of extraction procedures. Soil Science Society of America Journal, 51, 1515–1521.
Deacon, J. W. (1984). The nature and practice of biological-control of plant-pathogens - cook, rj, baker, kf. Nature, 309(5970), 732. doi:10.1038/309732a0.
Delp, B. R., & Milholland, R. D. (1980). Evaluating strawberry fragaria-ananassa plants for resistance to colletotrichum-fragariae. Plant Disease, 64(12), 1071–1073.
Freeman, S., Shalev, Z., & Katan, J. (2002). Survival in soil of Colletotrichum acutatum and C-gloeosporioides pathogenic on strawberry. Plant Disease, 86(9), 965–970. doi:10.1094/pdis.2002.86.9.965.
Fries, L. L. M., Pacovsky, R. S., Safir, G. R., & Siqueira, J. O. (1997). Plant growth and arbuscular mycorrhizal fungal colonization affected by exogenously applied phenolic compounds. Journal of Chemical Ecology, 23(7), 1755–1767.
Garrido, C., Carbú, M., Fernández-Acero, F. J., Budge, G., Vallejo, I., Colyer, A., et al. (2007). Isolation and pathogenicity of Colletotrichum spp. causing anthracnose of strawberry in south west Spain. European Journal of Plant Pathology, 120(4), 409–415. doi:10.1007/s10658-007-9224-7.
Hao, W.-y., Ren, L.-x., Ran, W., & Shen, Q.-r. (2010). Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f.sp. niveum. Plant and Soil, 336(1–2), 485–497. doi:10.1007/s11104-010-0505-0.
He, C. N., Gao, W. W., Yang, J. X., Bi, W., Zhang, X. S., & Zhao, Y. J. (2008). Identification of autotoxic compounds from fibrous roots of Panax quinquefolium L. Plant and Soil, 318(1–2), 63–72. doi:10.1007/s11104-008-9817-8.
Huang, X. X., Bie, Z. L., & Huang, Y. (2010). Identification of autotoxins in rhizosphere soils under the continuous cropping of cowpea. Allelopathy Journal, 25(2), 383–392.
Huang, L. F., Song, L. X., Xia, X. J., Mao, W. H., Shi, K., Zhou, Y. H., et al. (2013). Plant-soil feedbacks and soil sickness: from mechanisms to application in agriculture. Journal of Chemical Ecology, 39(2), 232–242. doi:10.1007/s10886-013-0244-9.
Inderjit, & Mallik, A. U. (1996). The nature of interference potential of Kalmia angustifolia. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere, 26(11), 1899–1904.
Jilani, G., Mahmood, S., Chaudhry, A. N., Hassan, I., & Akram, M. (2008). Allelochemicals: sources, toxicity and microbial transformation in soil - a review. Annals of Microbiology, 58(3), 351–357.
Kitazawa, H., Asao, T., Ban, T., Pramanik, M. H. R., & Hosoki, T. (2005). Autotoxicity of root exudates from strawberry in hydroponic culture. Journal of Horticultural Science & Biotechnology, 80(6), 677–680.
Kong, C. H., Wang, P., Zhao, H., Xu, X. H., & Zhu, Y. D. (2008). Impact of allelochemical exuded from allelopathic rice on soil microbial community. Soil Biology and Biochemistry, 40(7), 1862–1869. doi:10.1016/j.soilbio.2008.03.009.
Larry, L. S. (2008). Integrated Pest Management for Strawberries. California: UC IPM.
Lorenzo, P., Pereira, C. S., & Rodríguez-Echeverría, S. (2013). Differential impact on soil microbes of allelopathic compounds released by the invasive Acacia dealbata Link. Soil Biology and Biochemistry, 57, 156–163. doi:10.1016/j.soilbio.2012.08.018.
MacKenzie, S. J., Legard, D. E., Timmer, L. W., Chandler, C. K., & Peres, N. A. (2006). Resistance of strawberry cultivars to crown rot caused by Colletotrichum gloeosporioides isolates from Florida is nonspecific. [Article]. Plant Disease, 90(8), 1091–1097. doi:10.1094/pd-90-1091.
Nazih, N., Finlay-Moore, O., Hartel, P. G., & Fuhrmann, J. J. (2001). Whole soil fatty acid methyl ester (FAME) profiles of early soybean rhizosphere as affected by temperature and matric water potential. Soil Biology & Biochemistry, 33(4–5), 693–696. doi:10.1016/s0038-0717(00)00197-8.
Nemec, S. (1976). Response of three root rot fungi to strawberry phenolics and the relation of phenolics to disease resistance. Mycopathologia, 59(1), 37–40. doi:10.1007/BF00491202.
Prithiviraj, B., Perry, L. G., Badri, D. V., & Vivanco, J. M. (2007). Chemical facilitation and induced pathogen resistance mediated by a root-secreted phytotoxin. The New Phytologist, 173(4), 852–860. doi:10.1111/j.1469-8137.2006.01964.x.
Ren, L., Su, S., Yang, X., Xu, Y., Huang, Q., & Shen, Q. (2008). Intercropping with aerobic rice suppressed Fusarium wilt in watermelon. Soil Biology and Biochemistry, 40(3), 834–844. doi:10.1016/j.soilbio.2007.11.003.
Salazar, S. M., Castagnaro, A. P., Arias, M. E., Chalfoun, N., Tonello, U., & Ricci, J. C. D. (2007). Induction of a defense response in strawberry mediated by an avirulent strain of Colletotrichum. European Journal of Plant Pathology, 117(2), 109–122. doi:10.1007/s10658-006-9075-7.
Shi, S., Richardson, A. E., O’Callaghan, M., DeAngelis, K. M., Jones, E. E., Stewart, A., et al. (2011). Effects of selected root exudate components on soil bacterial communities. FEMS Microbiology Ecology, 77(3), 600–610. doi:10.1111/j.1574-6941.2011.01150.x.
Weir, T. L., Park, S. W., & Vivanco, J. M. (2004). Biochemical and physiological mechanisms mediated by allelochemicals. Current Opinion in Plant Biology, 7(4), 472–479. doi:10.1016/j.pbi.2004.05.007.
Widiastuti, A., Yoshino, M., Saito, H., Maejima, K., Zhou, S. Y., Odani, H., et al. (2013). Heat shock-induced resistance in strawberry against crown rot fungus Colletotrichum gloeosporioides. [Article]. Physiological and Molecular Plant Pathology, 84, 86–91. doi:10.1016/j.pmpp.2013.08.003.
Wu, H.-S., Raza, W., Fan, J.-Q., Sun, Y.-G., Bao, W., & Shen, Q.-R. (2008a). Cinnamic acid inhibits growth but stimulates production of pathogenesis factors by in vitro cultures of Fusarium oxysporum f.sp niveum. Journal of Agricultural and Food Chemistry, 56(4), 1316–1321. doi:10.1021/jf0726482.
Wu, H. S., Raza, W., Fan, J. Q., Sun, Y. G., Bao, W., Liu, D. Y., et al. (2008b). Antibiotic effect of exogenously applied salicylic acid on in vitro soilborne pathogen, Fusarium oxysporum f.sp.niveum. Chemosphere, 74(1), 45–50. doi:10.1016/j.chemosphere.2008.09.027.
Wu, H.-s., Wang, Y., Bao, W., Liu, D.-y., Raza, W., Huang, Q.-w., et al. (2009a). Responses of Fusarium oxysporum f. sp. niveum to exogenously added sinapic acid in vitro. Biology and Fertility of Soils, 45(4), 443–447. doi:10.1007/s00374-009-0353-3.
Wu, H.-S., Wang, Y., Zhang, C.-Y., Bao, W., Ling, N., Liu, D.-Y., et al. (2009b). Growth of in vitro Fusarium oxysporum f. sp niveum in chemically defined media amended with gallic acid. Biological Research, 42(3), 297–304.
Wu, H.-s., Luo, J., Raza, W., Liu, Y.-x., Gu, M., Chen, G., et al. (2010a). Effect of exogenously added ferulic acid on in vitro Fusarium oxysporum f. sp. niveum. Scientia Horticulturae, 124(4), 448–453. doi:10.1016/j.scienta.2010.02.007.
Wu, H. S., Liu, Y. D., Yang, X. I., Chen, X. Q., Wang, Z. H., Kong, X. Y., et al. (2010b). Growth responses of in vitro Fusarium oxysporum f. sp. niveum to external supply of tannic acid. Journal of Environmental Biology, 31(6), 1017–1022.
Xu, Y., Wang, G., Jin, J., Liu, J., Zhang, Q., & Liu, X. (2009). Bacterial communities in soybean rhizosphere in response to soil type, soybean genotype, and their growth stage. Soil Biology and Biochemistry, 41(5), 919–925. doi:10.1016/j.soilbio.2008.10.027.
Ye, S. F., Yu, J. Q., Peng, Y. H., Zheng, J. H., & Zou, L. Y. (2004). Incidence of Fusarium wilt in Cucumis sativus L. is promoted by cinnamic acid, an autotoxin in root exudates. Plant and Soil, 263(1–2), 143–150. doi:10.1023/B:Plso.0000047721.78555.Dc.
Yu, J. Q., & Matsui, Y. (1994). Phytotoxic substances in root exudates of cucumber (Cucumis-sativus l). Journal of Chemical Ecology, 20(1), 21–31. doi:10.1007/bf02065988.
Yu, J., & Yoshihisa, M. (1999). Autointoxication of root exudates in Pisum sativus. Acta Horticulturae Sinica, 26(3), 175–179.
Yu, J., Sun, Y., Zhang, Y., Ding, J., Xia, X., Xiao, C., et al. (2009). Selective trans-cinnamic acid uptake impairs [Ca2+]cyt homeostasis and growth in Cucumis sativus L. Journal of Chemical Ecology, 35(12), 1471–1477. doi:10.1007/s10886-009-9726-1.
Zhao, X., Zhen, W., Qi, Y., Liu, X., & Yin, B. (2009). Coordinated effects of root autotoxic substances and Fusarium oxysporum Schl. f. sp. fragariae on the growth and replant disease of strawberry. Frontiers of Agriculture in China, 3(1), 34–39. doi:10.1007/s11703-009-0006-1.
Zhou, X., & Wu, F. (2012a). Effects of amendments of ferulic acid on soil microbial communities in the rhizosphere of cucumber (Cucumis sativus L.). European Journal of Soil Biology, 50, 191–197. doi:10.1016/j.ejsobi.2012.03.001.
Zhou, X., & Wu, F. (2012b). p-Coumaric acid influenced cucumber rhizosphere soil microbial communities and the growth of Fusarium oxysporum f.sp. cucumerinum Owen. PloS One, 7(10), e48288. doi:10.1371/journal.pone.0048288.
Zhou, X., & Wu, F. (2013). Artificially applied vanillic acid changed soil microbial communities in the rhizosphere of cucumber (Cucumis sativusL.). Canadian Journal of Soil Science, 93(1), 13–21. doi:10.4141/cjss2012-039.
Zhou, X., Yu, G., & Wu, F. (2012a). Responses of soil microbial communities in the rhizosphere of cucumber (Cucumis sativus L.) to exogenously applied p-hydroxybenzoic acid. Journal of Chemical Ecology, 38(8), 975–983. doi:10.1007/s10886-012-0156-0.
Zhou, X., Yu, G., & Wu, F. (2012b). Soil phenolics in a continuously mono-cropped cucumber (Cucumis sativus L.) system and their effects on cucumber seedling growth and soil microbial communities. European Journal of Soil Science, 63(3), 332–340. doi:10.1111/j.1365-2389.2012.01442.x.
Acknowledgments
This work was supported by College PhD Subject Science & Technology Programme of the Education Ministry (20110008130003), the Commonwealth Science & Technology Programme of the Agricultural Ministry (201003064), and Chinese National Science & Technology Pillar Programme (30971978). The authors also thank Prof. Zhang Guozhen for providing strawberry C. gloeosporioides. We additionally thank the editors and reviewers for their valuable suggestions to improve this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Geilin Tian and Yanmeng Bi contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tian, G., Bi, Y., Sun, Z. et al. Phenolic acids in the plow layer soil of strawberry fields and their effects on the occurrence of strawberry anthracnose. Eur J Plant Pathol 143, 581–594 (2015). https://doi.org/10.1007/s10658-015-0711-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0711-y