Abstract
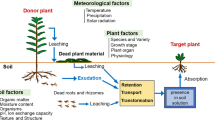
Soil microorganisms interact with plants in diversified manner ranging from mobilising nutrients and enhancing their growth, to inducing diseases. They also produce allelochemicals directly or indirectly through conversion from other compounds. In order to hamper plant growth, allelochemicals must accumulate and persist at phytotoxic levels in the rhizosphere soil. However, after their entry into environment, persistence, availability and biological activities of allelochemicals are influenced by microorganisms. Transformation of allelochemicals by soil microbes may result into the compounds with modified biological properties. Such bio-transformations affect the overall allelopathic capability of the producer plant in a direct manner. Several reports describe the allelopathic significance of microbial metabolism products. For instance, a bacteriumActinetobacter calcoaceticus, can convert 2 (3H)-benzoxazolinone (BOA) to 2,2′-oxo-l,l′-azobenzene (AZOB) which is more inhibitory to some plants. On the contrary, bacteriumPseudomonas putida catabolises juglone in soils beneath walnut trees; otherwise, juglone accumulates at phytotoxic levels. This review article describes the nature of microbially produced allelochemicals, and the ways to mediate microbial degradation of putative allelochemicals. The given information develops an understanding of persistence, fate and phytotoxicity of allelochemicals in the natural environment, and also points out the possible solution of the problems due to microbial interventions in the soil.
Similar content being viewed by others
References
Ahmad R., Jilani G., Arshad M., Zahir Z.A., Khalid A. (2007). Bioconversion of organic wastes for their recycling in agriculture: An overview of perspectives and prospects. Ann. Microbiol., 57 (4): 471–479.
Al-Turki A.I., Dick W.A. (2003). Myrosinase activity in soil. Soil Sci. Soc. Am. J., 67: 139–145.
An M., Johnson I.R., Lovett J.V. (1996). Mathematical modeling of allelopathy: I. Phytotoxicity caused by plant residues during decomposition. Allelopathy J., 3: 33–42.
An M., Pratley J.E., Ha T. (2000). Phytotoxicity ofVulpia residues: iv. Dynamics of allelochemicals during decomposition of vulpia residues and their corresponding phytotoxicity. J. Chem. Ecol., 26 (11): 2603–2617.
An M., Johnson I.R., Lovett J.V. (2002). Mathematical modelling of residue allelopathy: the effects of intrinsic and extrinsic factors. Plant Soil, 246: 11–22.
Bais H., Walker T., Stermitz F., Hufbauer R., Vivanco J. (2002). Enantiomeric-dependent phytotoxic and antimicrobial activity of (+/−)-catechin. A rhizosecreted racemic mixture from Spotted Knapweed. Plant Physiol., 128: 1173–1179.
Bais H.P., Vapachedu R., Gilroy S., Callaway R., Vivanco J. (2003). Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science, 301: 1377–1380.
Baumeler A., Hesse M., Werner C. (2000). Benzoxazinoids-cyclic hydroxamic acids, lactams and their corresponding glucosides in the genusAphelandra (Acanthaceae). Phytochemistry, 53: 213–222.
Bertin C., Yang X., Weston L.A. (2003). The role of root exudates and allelochemicals in the rhizosphere. Plant Soil, 256: 67–83.
Blum U. (1998). Effects of microbial utilization of phenolic acids and their phenolic acid breakdown products on allelopathic interactions. J. Chem. Ecol., 24: 685–708.
Blum U., Shafer S.R., Lehman M.E. (1999). Evidence for inhibitory allelopathic interactions involving phenolic acids in field soils: Concepts vs. an experimental model. Crit. Rev. Plant Sci., 18: 673–693.
Carballeira A., Carral E., Reigosa M.J. (1988). Asymmetric small-scale distribution and allelopathy: Interaction betweenRumex obtusifolius L. and meadow species. J. Chem. Ecol., 14: 1775–1786.
Chiapusio G., Pellissier F., Gallet C. (2004). Uptake and translocation of phytochemical 2-benzoxazolinone (BOA) in radish seeds and seedlings. J. Exp. Bot., 55 (402): 1587–1592.
Chou C.H., Lin H.J. (1976). Autointoxication mechanism ofOryza sativa. I. Phytotoxic effects of decomposing rice residues in soil. J. Chem. Ecol., 2: 353–367.
Dalton B.R. (1999). The occurrence and behavior of plant phenolic acids in soil environments and their potential involvement in allelochemical interference interactions: Methodological limitations in establishing conclusive proof of allelopathy. In: Inderjit, Dakshini K.M.M., Foy C.L., Eds, Principles and Practices in Plant Ecology: Allelochemical Interactions, CRC Press, Boca Raton, FL, pp. 57–74.
Dalton B.R., Blum U., Weed S.B. (1989). Differential sorption of exogenously applied ferulic, p-courmaric, p-hydroxybenzoic, and vanillic acids in soil. Soil Sci. Soc. Am. J., 53: 757–762.
Dornbos D.L. Jr, Spencer G.F., Miller R.W. (1990). Merdicarpin delays alfalfa seed germination and seedling growth. Crop Sci., 30: 162–166.
Elijarrat E., Barcelo D. (2001). Sample handling and analysis of allelochemical compounds in plants. Trends Anal. Chem., 20: 584–590.
Etzerodt T., Nielsen S.T., Mortensen A.G., Christophersen C., Fomsgaard I.S. (2006). Elucidating the transformation pattern of the cereal allelochemical 6-methoxy-2-benzoxazolinone (MBOA) and the trideuterio methoxy analogue [D3]-MBOA in soil. J. Agri. Food Chem., 54: 1075–1085
Feucht W., Treutter D. (1999). The role of flavan-3-ols and proanthocyanidins in plant defence. In: Inderjit, Dakshini K.M.M., Foy C.L., Eds, Principles and Practices in Plant Ecology: Allelochemical Interactions, CRC Press, Boca Raton, FL, pp. 307–338.
Fomsgaard I.S., Mortensen A.G., Gents M.B., Understrup A.G. (2004). Time-dependent transformation of varying concentrations of the hydroxamic acid metabolites MBOA and BOA in soil. In: Proceedings of Second European Allelopathy Symposium. Allelopathy — from Understanding to Application, FATEALLCHEM Workshop “Fate and Toxicity of Allelochemicals (Natural Plant Toxins) in Relation to Environment and Consumer”, June 3–5; Institute of Soil Science and Plant Cultivation Press Services, Pulawy, Poland, pp. 61–63.
Fomsgaard I.S., Mortensen A.G., Idinger J., Coja T., Blümel S. (2006). Transformation of benzoxazinones and derivatives and microbial activity in the test environment of soil ecotoxicological tests onPoecilus cupreus andFolsomia candida. J. Agri. Food Chem., 54: 1086–1092.
Friebe A., Wieland I., Schulz M. (1996). Tolerance ofAvena sativa to the allelochemical benzoxazolinone. Degradation of BOA by root-colonizing bacteria. J. Appl. Bot. — Angew. Bot., 70: 150–154.
Friebe A., Vilich V., Hennig L., Kluge M., Sicker D. (1998). Detoxification of benzoxazolinone allelochemicals from wheat byGaeumannomyces graminis var.tritici, G. graminis var.graminis, G. graminis var.avenae, andFusarium culmorum. Appl. Environ. Microbiol., 64: 2386–2391.
Gagliardo R.W., Chilton W.S. (1992). Soil transformation of 2(3H)-benzoxazolone of rye into phytotoxic 2-amino-3H-phenoxazin-3-one. J. Chem. Ecol., 18: 1683–1691.
Gents M.B., Mortensen A.G., Nielsen S.T., Christoffersen C., Fomsgaard I.S. (2005). Transformation products of 2-benzoxazolinone (BOA) in soil. Chemosphere, 61 (1): 74–84.
Giuntini E., Bazzicalupo M., Castaldini M., Fabiani A., Miclaus N., Piccolo R., Ranalli G., Santomassimo F., Zanobini S., Mengoni A. (2006). Genetic diversity of dinitrogen-fixing bacterial communities in soil amended with olive husks. Ann. Microbiol., 56 (2): 83–88.
Glenn A.E., Hinton D.M., Yates I.E., Bacon C.W. (2001). Detoxification of corn antimicrobial compounds as the basis for isolatingFusarium verticillioides and some otherFusarium species from corn. Appl. Environ. Microbiol., 67: 2973–2981.
Gries G. (1942). Juglone, the active agent in walnut toxicity. National Nut Growers Association Annual Report, 33: 52–55.
Hammond-Kosack K.E., Jones J.D.G. (1996). Resistance gene-dependent plant defense responses. Plant Cell, 8 (10): 1773–1791.
Haramoto E.R. (2004). The effects of brassica cover crops on weed dynamics. M.Sc. Thesis. Department of Plant, Soil, and Environmental Sciences, The University of Maine.
Harborne J.B., Ed. (1989). Plant phenolics. Methods in Plant Biochemistry No. 1, Academic Press, London.
Hong N.H., Xuan T.D., Tsuzuki E., Terao H., Matsuo M., Khanh T.D. (2004). Weed control of four higher plant species in paddy rice fields in southeast Asia. J. Agron. Crop Sci., 190: 59–64.
Huang P.M., Wang M.C., Wang M.K. (1999). Catalytic transformation of phenolic compounds in the soils. In: Inderjit, Dakshini K.M.M., Foy C.L., Eds, Principles and Practices in Plant Ecology: Allelochemical Interactions, CRC Press, Boca Raton, FL, pp. 287–306.
Inbaraj J.J., Chignell C.F. (2004). Cytotoxic action of juglone and plumbagin: a mechanistic study using HaCaT keratinocytes. Chem. Res. Toxicol., 17 (1): 55–62.
Inderjit, Dakshini K.M.M., Einhellig F.A. (1995). Allelopathy: Organisms, Processes and Applications, ACS Symposium Series 582. American Chemical Society, Washington, DC.
Inderjit (1996). Plant phenolics in allelopathy. Bot. Rev., 62 (2): 186–202.
Inderjit, Dakshini K.M.M. (1999). Bioassey for allelopathy: Interactions of soil organic and inorganic constituents. In: Inderjit, Dakshini K.M.M., Foy C.L., Eds, Principles and Practices in Plant Ecology: Allelochemical Interactions, CRC Press, Boca Raton, FL, pp. 35–44.
Inderjit (2001). Soil environment effects on allelochemical activity. Agron. J., 93: 79–84.
Inderjit, Weston L.A. (2003). Root exudation: an overview. In: de Kroon, Visser, E.J.W., Eds, Root Ecology, Springer-Verlag, Heidelberg, Germany.
Jilani G., Akram A., Ali R.M., Hafeez F.Y., Shamsi I.H., Chaudhry A.N., Chaudhry A.G. (2007). Enhancing crop growth, nutrients availability, economics and beneficial rhizosphere microflora through organic and biofertilizers. Ann. Microbiol., 57 (2): 177–183.
Kamei H., Koide T., Kojima T., Hashimoto Y., Hasegawa M. (1998). Inhibition of cell growth in culture by quinones. Cancer Biother. Radiopharm., 13 (2): 185–188.
Kara O., Asan A. (2007). Microfungal community structure from forest soils in Northern Thrace Region, Turkey. Ann. Microbiol., 57 (2): 149–155.
Kohli R.K., Singh H.P., Batish D.R. (2001). Allelopathy in Agroecosystems. Food Products Press, New York.
Kong C., Liang W., Hu F., Xu X., Wang P., Jiang Y., Xing B. (2004). Allelochemicals and their transformations in theAgeratum conyzoides intercropped citrus orchard soils. Plant Soil, 264: 149–157.
Kumar P., Gagliardo R.W., Chilton W.S. (1993). Soil transformation of wheat and corn metabolites MBOA and DIM2BOA into aminophenoxazinones. J. Chem. Ecol., 19: 2453–2461.
Lee K.C., Campbell R.W. (1969). Nature and occurrence of juglone inJuglans nigra. Hort. Sci., 4: 297–298.
Lehmann R.G., Cheng H.H., Harsh J.B. (1987). Oxidation of phenolic acids by soil iron and manganese oxides. Soil Sci. Soc. Am. J., 51: 352–356.
Macías F.A., Oliveros-Bastidas A., Marían D., Castellano D., Simonet A.M., Molinillo J.M.G. (2004). Degradation studies on benzoxazinoids. Soil degradation dynamics of 2,4-Dihydroxy-7-methoxy-(2H)-1,4-benzoxazin-3(4H)-one (DIMBOA) and its degradation products, phytotoxic allelochemicals from Gramineae. J. Agri. Food Chem., 52: 6402–6413.
Makino T., Takahashi Y., Sakurai Y., Nanzyo M. (1996). Influence of soil chemical properties on adsorption and oxidation of phenolic acids in soil suspension. Soil Sci. Plant Nutr., 42: 867–879.
Mason-Sedun W., Jessop R.S. (1988). Differential phytotoxicity among species and cultivars of the genusBrassica to wheat. II. Activity and persistence of water-soluble phytotoxins from residues of the genusBrassica. Plant Soil, 107: 69–80.
Molísch H. (1937). Der Einfluss einer Pflanze auf die andere-Allelopathie. Fischer, Jena (In German).
Morimoto M., Komai K. (2005). Plant growth inhibitors: Patchoulane-type sesquiterpenes fromCyperus rotundus L. Weed Biol. Manag., 5: 203–209.
Nakahisa K., Tsuzuki E., Terao H., Koseinura S. (1994). Study on the allelopathy of alfalfa (Medicago sativa L.). 2. Isolation and identification of allelopathic substance in alfalfa. Japanese 9. Crop Sci., 63: 278–284.
Niemeyer H.M. (1988). Hydroxamic acids (4-hydroxy-1,4-benzoxazin-3-ones), defence chemicals in the Gramineae. Phytochemistry, 27: 3349–3358.
Ohno T., First P.R. (1998). Assessment of the Folin and Ciocalteu’s method for determining soil phenolic carbon. J. Environ. Qual., 27: 776–782.
Ohno T., Doolan K., Zibilske L.M., Liebman M., Gallandt E.R., Berube C. (2000). Phytotoxic effects of red clover amended soils on wild mustard seedling growth. Agri. Ecosyst. Environ., 78: 187–192.
Okumura M., Filonow A.B., Waller G.R. (1999). Use of14C-labeled alfalfa saponins for monitoring their fate in soil. J. Chem. Ecol., 25: 2575–2583.
Osbourn A.E. (1999). Antimicrobial phytoprotectants and fungal pathogens: a commentary. Fungal Genet. Biol., 26: 163–168.
Patrick Z.A., Toussoun T.A., Snyder W.C. (1963). Phytotoxic substances in arable soils associated with decomposition of plant residues. Phytopathology, 53: 152–161.
Pramanik M.H.R., Nagal M., Asao M., Matsui Y. (2000). Effect of temperature amd photoperiod on phytotoxic root exudates of cucumber (Cucumis sativus) in hydroponic culture. J. Chem. Ecol., 26: 1953–1967.
Qasem J.R., Foy C.L. (2001). Weed allelopathy, its ecological impacts and future prospects: a review. J. Crop Prod., 4: 43–92.
Rasmussen J.A., Einhellig F.A. (1977). Synergistic inhibitory effects of p-coumaric and ferulic acids on germination and growth of grain sorghum. J. Chem. Ecol., 3: 197–205.
Ren H., Song T., Wu T.Q., Sun L., Liu Y.X., Yang F., Chen Z.Y., Dong H. (2006). Effects of a biocontrol bacterium on growth and defence of transgenic rice plants expressing a bacterial type-III effector. Ann. Microbiol. 56 (4): 281–287.
Rice E.L. (1984). Allelopathy, 2nd edn., Academic Press, N.Y., USA.
Sampietro D.A., Vattuone M.A. (2006). Nature of the interference mechanism of sugarcane (Saccharum officinarum L.) straw. Plant Soil, 280: 157–169.
Schmidt S.K., Ley R.E. (1999). Microbial competition and soil structure limit the expression of allelochemicals in nature. In: Inderjit, Dakshini K.M.M., Foy C.L., Eds, Principles and Practices in Plant Ecology: Allelochemical Interactions, CRC Press, Boca Raton, FL, pp. 339–351.
Shaukat S.S., Siddiqui I.A., Khan G.H., Zaki M.J. (2002). Nematicidal and allelopathic potential ofArgemone mexicana, a tropical weed. Plant Soil, 245: 239–247.
Shaw L.J., Morris P., Hooker J.E. (2006). Perception and modification of plant flavonoid signals by rhizosphere microorganisms. Environ. Microbiol., 8 (11): 1867–1880.
Shindo H., Kuwatsuka S. (1975a). Behavior of phenolic substances in the decaying process of plants: II. Changes of phenolic substances in the decaying process of rice straw under various conditions. Soil Sci. Plant Nutr., 21: 215–225.
Shindo H., Kuwatsuka S. (1975b). Behavior of phenolic substances in the decaying process of plants: III. Degradation pathway of phenolic acids. Soil Sci. Plant Nutr., 21: 227–238.
Tang C.S., Waiss A.C. (1978). Short-chain fatty acids as growth inhibitors in decomposing wheat straw. J. Chem. Ecol., 4: 225–232.
Testa B. (1995). The Metabolism of Drugs and other Xenobiotics: Biochemistry of Redox Reactions, Academic Press, New York.
Tsuzuki E. (2001). Application of buckwheat as a weed control. Agri. Hort., 76: 55–62 (in Japanese).
Understrup A.G., Ravnskov S., Hansen H.C.B., Fomsgaard I.S. (2005). Biotransformation of 2-benzoxazolinone to 2-amino-(3H)-phenoxazin-3-one and 2-acetylamino-(3H)-phenoxazin-3-one in soil. J. Chem. Ecol., 31 (5): 1205–1222.
Van Etten H.D., Sandrock R.W., Wasmann C.C., Soby S.D., McCluskey K., Wang P. (1995). Detoxification of phytoanticipins and phytoalexins by phytopathogenic fungi. Can. J. Bot., 73: 518–525.
Vilich V., Löhndorf B., Sikora R.A., Friebe A. (1999). Metabolism of benzoxazolinone allelochemicals ofZea mays byFusarium subglutinans. Mycol. Res., 103: 1529–1532.
Virtanen A.I., Hietala P.K. (1960). Precursors of benzoxazolinone in rye plants. Acta Chem. Scand., 14: 499–504.
Weidenhamer J.D., Romeo J.T. (2004). Allelochemicals ofPolygonella myriophylla: Chemistry and soil degradation. J. Chem. Ecol., 30 (5): 1067–1082.
Weir T.L., Park S.W., Vivanco J.M. (2004). Biochemical and physiological mechanisms mediated by allelochemicals. Curr. Opin. Plant Biol., 7: 472–479.
Weston L.A., Nimbal C.H., Jeandet P. (1999). Allelopathic potential of grain sorghum (Sorghum bicolor (L.) Moench) and related species. In: Inderjit, Dakshini K.M.M., Foy C.L., Eds, Principles and Practices in Plant Ecology: Allelochemical Interactions, CRC Press, Boca Raton, FL, pp. 467–477.
Williamson G.B., Weidenhamer J.D. (1990). Bacterial degradation of juglone: Evidence against allelopathy. J. Chem. Ecol., 16 (5): 1739–1742.
Xuan T.D., Tsuzuki E. (2002). Varietal difference in allelopathic potential of alfalfa (Medicago sativa L.). J. Agro. Crop Sci., 188: 2–7.
Xuan T.D., Chikara J., Ogushi Y., Tsuzuki E., Terao H., Khanh T.D., Matsuo M. (2003a). Application of kava (Piper methysticum L.) root as potential herbicide and fungicide. Crop Prot., 22: 873–881.
Xuan T.D., Tsuzuki E., Matsuo M., Khanh T.D. (2003b). Correlation between inhibitory exhibition and suspected allelochemicals in alfalfa (Medicago sativa L.). Plant Prod. Sci., 6: 165–171.
Xuan T.D., Tsuzuki E., Terao H., Matsuo M., Khanh T.D. (2003c). Identification of potential allelochemicals in kava (Piper methysticum L.) root. Allelopathy J., 12, 197–204.
Xuan T.D., Tawata S., Khanh T.D., Chung I.M. (2005). Decomposition of allelopathic plants in soil. J. Agron. Crop Sci., 191: 162–171.
Yue Q., Bacon C.W., Richardson M.D. (1998). Biotransformation of 2-benzoxazolinone and 6-methoxy-benzoxazolinone byFusarium moniliforme. Phytochemistry, 48: 451–454.
Zikmundová M., Drandarov K., Bigler L., Hesse M., Werner C. (2002a). Biotransformation of 2-benzoxazolinone and 2-hydroxy-1,4-benzoxazin-3-one by endophytic fungi isolated fromAphelandra tetragona. Appl. Environ. Microbiol., 68 (10): 4863–4870.
Zikmundová M., Drandarov K., Hesse M., Werner C. (2002b). Hydroxylated 2-amino- 3H-phenoxazin-3-one derivatives as products of 2-hydroxy-1,4-benzoxazin-3-one (HBOA) biotransformation byChaetosphaeria sp., an endophytic fungus fromAphelandra tetragona. Z. Naturforsch, 57c: 660–665.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jilani, G., Mahmood, S., Chaudhry, A.N. et al. Allelochemicals: sources, toxicity and microbial transformation in soil —a review. Ann. Microbiol. 58, 351–357 (2008). https://doi.org/10.1007/BF03175528
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03175528