Abstract
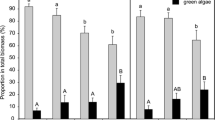
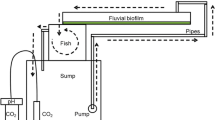
An indoor channel system was colonised with fluvial biofilms to study the chronic effects of high Fe and SO4 2− concentrations and acidic pH, the water chemistry in the surrounding streams of Aljustrel mining area (Alentejo, Portugal), and their contribution to community (in)tolerance to metal toxicity by short-term experiments with Cu and Zn. Biofilms were subjected to four different treatments during 8 weeks: high Fe and SO4 2− concentrations (1 mg Fe l−1 + 700 mg SO 2− 4 l−1) and acidic pH, high Fe and SO4 2− at alkaline pH; lower Fe and SO4 2− at acidic pH: and lower Fe and SO4 2− concentrations at alkaline pH as negative control. During chronic exposure, acidic pH affected growth negatively, based on low values of algal biomass and the autotrophic index, high values of the antioxidant enzyme activities and low diversity diatom communities, dominated by acidophilic species (Pinnularia aljustrelica) in acidic treatments, being the effects more marked with high Fe and SO4 2−. Co-tolerance to metals (Cu and Zn) was also shown in biofilms from the acidic treatments, contrasting with the higher sensitivity observed in the alkaline treatments. We can conclude that the Aljustrel mining area acidic environment limits algal growth and exerts a strong selection pressure on the community composition which is in turn, more tolerant to metal exposure.





Similar content being viewed by others
References
Baker BJ, Lutz MA, Dawson SC, Bond PL, Banfield JF (2004) Metabolically active eukaryotic communities in extremely acidic mine drainage. Appl Environ Microbiol 70(10):6264–6271
Barbour MT, Gerritsen J, Snyder BD, Stribling JB (1999) Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish, 2nd edn. EPA 841-B-99-002. US Environmental Protection Agency, Office of Water, Washington, DC
Bennett HD (1969) Algae in relation to mine water. Castanea 34:306–328
Bonet B, Corcoll N, Acuňa V, Sigg L, Behra R, Guasch H (2013) Seasonal changes in antioxidant enzyme activities of freshwater biofilms in a metal polluted Mediterranean stream. Sci Tot Environ 444:60–72
Bonnineau C, Bonet B, Corcoll N, Guasch H (2011) Catalase in fluvial biofilms: a comparison between different extraction methods and example of application in a metal-polluted river. Ecotoxicol 20:293–303
Clements WH, Newman MC (2002) Community Ecotoxicology. John Wiley, Chichester
Corcoll N, Bonet B, Leira M, Guasch H (2011) Chl-a fluorescence parameters as biomarkers of metal toxicity in fluvial biofilms: an experimental study. Hydrobiologia 673:119–136
Das BK, Roy A, Koschorreck M, Mandal SM, Wendt-Potthoff K, Bhattacharya J (2009) Occurrence and role of algae and fungi in acid mine drainage environment with special reference to metals and sulfate immobilization. Water Res 43:883–894
Ferreira da Silva E, Almeida SFP, Nunes ML, Luís AT, Borg F, Hedlund M, Marques de Sá C, Patinha C, Teixeira P (2009) Heavy metal pollution downstream the abandoned Coval da Mó mine (Portugal) and associated effects on epilithic diatom communities. Sci Total Environ 407:5620–5636
Foster PL (1982) Species associations and metal contents of algae from rivers polluted by heavy metals. Freshwater Biol 12:17–39
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133(1):21–25
Gaur JP, Rai LC (2001) Heavy metal tolerance in algae. In: Rai LC et al (eds) Algal adaptation to environmental stresses. Springer, Berlin, pp 363–388
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990(1):87–92
Gold C, Feurtet-Mazel A, Coste M, Boudou A (2003) Impacts of Cd and Zn on the development of periphytic diatom communities in artificial streams located along a river pollution gradient. Arch Environ Contam Toxicol 44(2):189–197
González-Toril E, Llobet-Brossa E, Casamayor EO, Amann R, Amils R (2003) Microbial ecology of an extreme acidic environment, the Tinto River. Appl Environ Microbiol 69(8):4853–4865
Gross S, Robbins EI (2000) Acidophilic and acid-tolerant fungi and yeasts. Hydrobiologia 433:91–109
Guasch H, Paulsson M, Sabater S (2002) Effect of copper on algae communities from oligotrophic calcareous streams. J Phycol 38(2):241–248
Guasch H, Atli G, Bonet B, Corcoll N, Leira M, Serra A (2010) Discharge and the response of biofilms to metal exposure in Mediterranean rivers. Hydrobiologia 657:143–157
Guasch H, Acosta XG, Urrea G, Bañeras L (2012) Changes in the microbial communities along the environmental gradient created by a small Fe spring. Freshwater Sci 31(2):599–609
Hall RJ, Likens GE, Fiance SB, Hendrey GR (1980) Experimental acidification of a stream In the Hubbard Brook Experimental Forest, New Hampshire. Ecology 61:976–989
Hamilton PB, Duthie HC (1984) Periphyton colonization of rock surfaces in a boreal forest stream studied by scanning electron microscopy and track autoradiography. J Phycol 20(4):525–532
Hargreaves JW, Lloyd EJH, Whitton BA (1976) Chemistry and vegetation of highly acidic streams. Freshwater Biol 5:563–576
Hendrey GR (1976) Effects of pH on the growth of periphytic algae in artificial stream channels (IR 25/76.SNSF project). SNSF, Ås-NLH, Oslo, pp 50
Ivorra N, Barranguet C, Jonker M, Kraak MHS, Admiraal W (2002) Metal-induced tolerance in the freshwater microbenthic diatom Gomphonemaparvulum. Environ Pollut 116:147–157
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pfl 167:191–194
Klapheck S, Fliegner W, Zimmer I (1994) Hydroxymethyl-Phytochelatins [γ-glutamylcysteine n-serine] are metal-induced peptides of the Poaceae. Plant Physiol 104(4):1325–1332
Krammer K, Lange-Bertalot H (1986) Bacillariophyceae, Naviculaceae, Süßwasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart, p 876
Krammer K, Lange-Bertalot H (1988) Bacillariophyceae, Bacillariaceae, Epithemiaceae, Surirellacea, Süßwasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart, p 596
Krammer K, Lange-Bertalot H (1991a) Bacillariophyceae, Centrales, Fragilariaceae, Eunoticeae, Süßwasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart, p 577
Krammer K, Lange-Bertalot H (1991b) Bacillariophyceae, Achnanthaceae, Kristische Ergänzungen zu Navicula (Lineolatae) und Gomphonema, Gesamtliteraturverzeichnis, Süßwasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart, p 437
Le Faucheur S, Bhera R, Sigg L (2005) Thiol metal contents in periphyton exposed to elevated copper and zinc concentrations: a field and microcosm study. Environ Sci Technol 39(20):8099–8107
Li M, Hu C, Zhu Q, Chen L, Kong Z, Liu Z (2006) Copper and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in the microalga Pavlova viridis (Prymnesiophyceae). Chemosphere 62(4):565–572
Lima A, Pereira S, Figueira E, Caldeira G, Caldeira H (2006) Cadmium detoxification in roots of Pisumsativum seedlings: relationship between toxicity levels, thiol pool alterations and growth. Environ Exp Bot 55:149–162
Luís AT, Novais MH, Van de Vijver B, Almeida SFP, Ferreira da Silva EA, Hoffmann L, Ector L (2012) Pinnularia aljustrelica sp. nov. (Bacillariophyceae), a new diatom species found in acidic waters in the Aljustrel mining area (Portugal) and further observations on the taxonomy, morphology and ecology of P. acidophila HOFMANN et KRAMMER and P. acoricola HUSTEDT. Fottea 12(1):27–40
Luís AT, Teixeira P, Almeida SFP, Ector L, Matos JX, Ferreira da Silva EA (2009) Impact of acid mine drainage (AMD) on water quality, stream sediments and periphytic diatom communities in the surrounding streams of Aljustrel mining area (Portugal). Water Air Soil Pollut 200:147–167
Mulholland PJ, Elwood JW, Palumbo AV, Stevenson RJ (1986) Effect of stream acidification on periphyton composition, chlorophyll, and productivity. Can J Fish Aquat Sci 43(10):1846–1858
Murphy J, Riley JP (1992) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Niyogi DK, Lewis WM, McKnight DM (2002) Effects of stress from mine drainage on diversity, biomass, and function of primary producers in mountain streams. Ecosystems 5:554–567
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant MolBiol 49:249–279
Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signaling. J Exp Bot 53(372):1283–1304
Nordstrom DK, Alpers CN (1999) Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund site. California. PNAS 96(7):3455–3462
Olaueson MM, Stokes PM (1989) Responses to the acidophilic alga Euglena mutabilis (Euglenophyceae) to carbon enrichment at pH 3. J Phycol 25(3):529–539
Pinto E, Sigaud-Kutner TCS, Leitão MAS, Okamoto OK, Morse D, Colepicolo P (2003) Heavy metal-induced oxidative stress in algae. J Phycol 39(6):1008–1018
Planas D (1996) Acidification effects. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: Freshwater benthic ecosystems. Academic, San Diego, CA, USA, pp 497–530
Purchase D, Miles RJ, Young TWK (1997) Cadmium uptake and nitrogen fixing ability in heavy-metal-resistant laboratory and field strains of Rhizobium leguminosarum biovar trifolii. FEMS Microbiol Ecol 22(1):85–93
Riseng CM, Gensemer RW, Kilham SS (1991) The effects of pH, aluminium, and chelator manipulations on the growth of acidic and circumneutral species of Asterionella. Water Air Soil Pollut 60:249–261
Rysgaard S, Kühl M, Glud RN, Hansen JW (2001) Biomass, production and horizontal patchiness of sea ice algae in a high-Arctic fjord (Young Sound, NE Greenland). Mar Ecol Prog Ser 223:15–26
Sabater S, Navarro E, Guasch H (2002) Effects of copper on algal communities at different current velocities. J Appl Phycol 14:391–398
Sabater S, Guasch H, Ricart M, Romaní A, Vidal G, Klünder C, Schmitt-Jansen M (2007) Monitoring the effect of chemicals on biological communities. The biofilm as an interface. Anal Bioanal Chem 387:1425–1434
Sabatini SE, Juarez AB, Eppis MR, Bianchi L, Luquet CM, Molina MCR (2009) Oxidative stress and antioxidant defenses in two green microalgae exposed to copper. Ecotoxicol Environ Saf 72(4):1200–1206
Serra A, Guasch H, Martí E, Geiszinger A (2009) Measuring in-stream retention of copper by means of constant-rate additions. Sci Total Environ 407(12):3847–3854
Skousen J, Sextone A, Garbutt K, Sencindiver J (1994) Acid mine drainage treatment with wetlands and anoxic limestone drains. In: Kent DM (ed) Applied wetland science and technology. Lewis, Boca Raton, pp 263–282
Sneller FE, van Heerwaarden LM, Koevoets PL, Vooijs R, Schat H, Verkleij JA (2000) Derivatization of phytochelatins from Silene vulgaris, induced upon exposure to arsenate and cadmium: comparison of derivatization with Ellman’s reagent and monobromobimane. J Agric Food Chem 48(9):4014–4019
Vanacker H, Carver TLW, Foyer CH (2000) Early H2O2 accumulation in mesophyll cells leads to induction of glutathione during the hyper-sensitive response in the barley-powdery mildew interaction. Plant Physiol 123(4):1289–1300
Verb RG, Vis ML (2000) Comparison of benthic diatom assemblages from streams draining abandoned and reclaimed coal mines and non-impacted sites. J N Am Benthol Soc 19:274–288
Acknowledgments
The authors are grateful to the Biology and Geosciences Departments of the University of Aveiro, Portugal, to the Fundação para a Ciência e Tecnologia, Portugal (grant number SFRH/BD/36137/2007) and to the University of Girona for their financial support by the project FLUVIALMULTISTRESS funded by the Spanish “Ministerio de Economía y competitividad” ref: CTM2009-14111-CO2-01. The authors also express their gratitude to the “ServeisTècnics de Recerca” of the University of Girona for offering their facilities and technical assistance during the metal analysis. We thank Ana Lima for doing the phytochelatins (PCs) and total gluthatione (GSH) analyses.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luís, A.T., Bonet, B., Corcoll, N. et al. Experimental evaluation of the contribution of acidic pH and Fe concentration to the structure, function and tolerance to metals (Cu and Zn) exposure in fluvial biofilms. Ecotoxicology 23, 1270–1282 (2014). https://doi.org/10.1007/s10646-014-1270-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1270-2