Summary
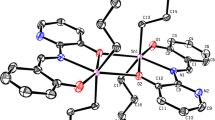
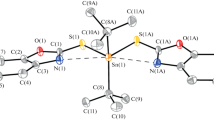
Potassium 2-{[(2Z)-(3-hydroxy-1-methyl-2-butenylidene)]amino}-4-methyl-pentanoate (L1HK) and potassium 2-{[(E)-1-(2-hydroxyphenyl)alkylidene]amino}-4-methyl-pentanoates (L2HK-L3HK) underwent reactions with PhnSnCl4-n (n = 2 and 3) to give the amino acetate functionalized Schiff base organotin(IV) complexes [Ph3SnLH] n (1–3) and [Ph2SnL] (4), respectively. These complexes have been characterized by 1H, 13C, 119Sn NMR, IR spectroscopic techniques in combination with elemental analyses. The crystal structures of 1 and 3 were determined. The crystal structures reveal that the complexes exist as polymeric chains in which the L-bridged Sn-atoms adopt a trans-R3SnO2 trigonal bipyramidal configuration with the Ph groups in the equatorial positions and the axial locations occupied by a carboxylate oxygen atom from one carboxylate ligand and the alcoholic or phenolic oxygen atom of the next carboxylate ligand in the chain. The carboxylate ligands coordinate in the zwitterionic form with the alcoholic/phenolic proton moved to the nearby nitrogen atom. The solution structures were predicted by 119Sn NMR spectroscopy. When these organotin(IV) complexes were tested against A498, EVSA-T, H226, IGROV, M19 MEL, MCF7 and WIDR human tumor cell lines, the average ID50 values obtained were 55, 80 and 35 ng/ml for triphenyltin(IV) compounds 1–3, respectively. The most cytotoxic triphenyltin(IV) compound in the present report (3) with an average ID50 value of around 35 ng/ml is found to be more cytotoxic for all the cell lines studied than doxorubicin, cisplatin, 5-fluorouracil and etoposide.




Similar content being viewed by others
References
Schwartsmann G, Ratain MJ, Cragg GM, Wong JE, Saijo N, Parkinson DR, Fujiwara Y, Pazdur R, Newman DJ, Dagher R, Di Leone L (2002) Anticancer drug discovery and development throughout the world. J Clin Oncol 20(Suppl 15):47S–59S
Neidle S, Thurston DE (2005) Chemical approaches to the discovery and development of cancer therapies. Nat Rev Cancer 5:285–296. doi:10.1038/nrc1587
Narayanan VL, Nasr M, Paul KD (1990) In: Gielen M (ed) Tin-based antitumor drugs. Springer, Berlin, pp 201–217
Crowe AJ (1988) In: Gielen M (ed) Metal based antitumour drugs, vol. 1. Freund, London, pp 103–149
Gielen M, Lelieveld P, de Vos D, Willem R (1992) In: Gielen M (ed) Metal based antitumour drugs, vol. 2. Freund, Tel Aviv, pp 29–54
Pellerito C, Agati PD, Fiore T, Mansueto C, Mansueto V, Stocco G, Nagy L, Pellerito L (2005) Synthesis, structural investigations on organotin(IV) chlorin-e6 complexes, their effect on sea urchin embryonic development and induced apoptosis. J Inorg Biochem 99:1294–1305. doi:10.1016/j.jinorgbio.2005.03.002
Cima F, Ballarin L (1999) TBT-induced apoptosis in tunicate haemocytes. Appl Organomet Chem 13:697–703. doi:10.1002/(SICI)1099-0739(199910)13:10<697::AID-AOC916>3.0.CO;2
Shuaibu MN, Kanbara H, Yanagi T, Ichinose A, Ameh DA, Bonire JJ, Nok AJ (2003) In vitro trypanocidal activity of dibutyltin dichloride and its fatty acid derivatives. Jpn Parasitolog Res 91:5–11. doi:10.1007/s00436-003-0861-2
Jan C-R, Jiann B-P, Lu Y-C, Chang H-T, Su W, Chen W-c, Yu C-C, Huang J-K (2002) Effect of the organotin compound triethyltin on Ca2+ handling in human prostate cancer cells. Life Sci 70:1337–1345. doi:10.1016/S0024-3205(01)01500-4
Samuel MP, de Vos D, Raveendra D, Sarma JARP, Roy S (2002) 3-D QSAR studies on new dibenzyltin(IV) anticancer agents by comparative molecular field analysis (CoMFA). Bioorg Med Chem Lett 12:61–64. doi:10.1016/S0960-894X(01)00684-9
Carraher CE Jr, Battin A, Shahi KR, Roner MR (2007) Synthesis, structural characterization, and initial evaluation as anticancer Drugs of dibutyltin polyamines derived from various 4,6-diaminopyrimidines. J Inorg Organomet Polym 17:631–639. doi:10.1007/s10904-007-9159-7
Barot G, Shahi KR, Roner MR, Carraher CE Jr (2007) Synthesis, structural characterization, and ability to Inhibit cancer growth of a series of organotin poly(ethylene glycols). J Inorg Organomet Polym 17:595–603. doi:10.1007/s10904-007-9158-8
Roner M, Carraher C, Sabir T, Shahi K, Roehr J, Bassett K (2006) Anticancer and antiviral activities of organotin polyether amines derived from the antiviral acyclovir. Polym Mater Sci Eng 95:525–527
Carraher C, Ashida Y, Battin G (2006) Synthesis of organotin polyethers containing diethylstilbestrol. Polym Mater Sci Eng 95:556–558
Carraher C, Sabir T, Roner M, Shahi K, Bleicher R, Roehr J, Bassett K (2006) Synthesis of organotin polyamine ethers containing acyclovir and their preliminary anticancer and antiviral activity. J Inorg Organomet Polym 16:249–257. doi:10.1007/s10904-006-9050-y
Roner M, Carraher C, Roehr J, Bassett K (2006) Antiviral and anticancer activity of organotin polymers and reactants derived from norfloxacin and ampicillin. J Polym Mater 23:153–159
Carraher C, Siegmann-Louda D (2004) Macromolecules containing metal and metal-like Elements, vol 3. Biomedical applications. Wiley, Hoboken, NJ
Doucette R, Siegmann-Louda D, Carraher C, Cardoso A (2004) Inhibition of Balb 3T3 cells as a function of metal for kinetin containing polymers. Polym Mater Sci Eng 91:564–566
Blower PJ (2004) Inorganic pharmaceuticals. Annu Rep Prog Chem Sect A 100:633–658. doi:10.1039/b312109g
Gielen M (2003) An overview of forty years organotin chemistry developed at the Free Universities of Brussels ULB and VUB. J Braz Chem Soc 14:870–877. doi:10.1590/S0103-50532003000600003
Höti N, Ma J, Tabassum S, Wang Y, Wu M (2003) Triphenyl tin benzimidazolethiol, a novel antitumor agent, induces mitochondrial-mediated apoptosis in human cervical cancer cells via suppression of HPV-18 encoded E6. J Biochem 134:521–528. doi:10.1093/jb/mvg169
Xanthopoulou MN, Hadjikakou SK, Hadjiliadis N, Schürmann M, Jurkschat K, Michaelides A, Skoulika S, Bakas T, Binolis J, Karkabounas S, Charalabopoulos K (2003) Synthesis, structural characterization and in vitro cytotoxicity of organotin(IV) derivatives of heterocyclic thioamides, 2-mercaptobenzothiazole, 5-chloro-2-mercaptobenzothiazole, 3-methyl-2-mercaptobenzothiazole and 2-mercaptonicotinic acid. J Inorg Biochem 96:425–434. doi:10.1016/S0162-0134(03)00178-8
Höti N, Zhu D-e, Song Z, Wu Z, Tabassum S, Wu M (2004) p53-dependent apoptotic mechanism of a new designer bimetallic compound tri-phenyl tin benzimidazolethiol copper chloride (TPT-CuCl2): In vivo studies in Wistar rats as well as in vitro studies in human cervical cancer cells. J Pharmacol Exp Ther 311:22–33. doi:10.1124/jpet.104.069104
Chasapis CT, Hadjikakou SK, Garoufis A, Hadjiliadis N, Bakas T, Kubicki M, Ming Y (2004) Organotin(IV) derivatives of L-cysteine and their in vitro anti-tumor properties. Bioinorg Chem Appl 2:43–54. doi:10.1155/S1565363304000044
Maher T, Snyder J, Durham P, Gerasimchuk NN (2005) Proceedings of the 229th ACS National Metting, San Diego, CA, United States, March 13-17, INOR-719 (abstracts)
Tabassum S, Pettinari C (2006) Chemical and biotechnological developments in organotin cancer chemotherapy. J Organomet Chem 691:1761–1766. doi:10.1016/j.jorganchem.2005.12.033
Gielen M, Tiekink ERT (eds) (2005) Metallotherapeutic drug and metal-based diagnostic agents: 50Sn Tin compounds and their therapeutic potential. Wiley, Chichester, England, pp 421–439 (and references therein)
Blunden SJ, Evans CJ (1990) In: Hutziger O (ed.) Anthropogenic compounds. Springer, Heidelberg, pp 1-44
Evans CJ (1998) In: Smith PJ (eds.) Chemistry of tin. Blackie Academic, London, pp 442–479
Willem R, Bouhdid A, Mahieu B, Ghys L, Biesemans M, Tiekink ERT, de Vos D, Gielen M (1997) Synthesis, characterization and in vitro antitumour activity of triphenyl- and tri-n-butyltin benzoates, phenylacetates and cinnamates. J Organomet Chem 531:151–158. doi:10.1016/S0022-328X(96)06686-7
Gielen M, El Khloufi A, Biesemans M, Bouhdid A, de Vos D, Mahieu B, Willem R (1994) Synthesis, characterization and high in vitro antitumour activity of novel triphenyltin carboxylates. Metal-Based Drugs 1:305–309. doi:10.1155/MBD.1994.305
Bouălam M, Gielen M, El Khloufi A, de Vos D, Willem R (1993) Novel organo-tin compounds having anti-tumour activity and anti-tumour compositions, Pharmachemie B.V., Eur Pat, Publ 538 517, Appl. 91/202, 746.3-, 22.10.91. Chem Abstr 119:117548b
Gielen M, Willem R, Biesemans M, Bouălam M, El Khloufi A, de Vos D (1992) Exceptionally high in vitro antitumour activity of substituted triphenyltin benzoates including salicylates against a human mammary tumour, MCF-7, and a colon carcinoma, WiDr. Appl Organomet Chem 6:287–291. doi:10.1002/aoc.590060307
Kemmer M, Gielen M, Biesemans M, de Vos D, Willem R (1998) Synthesis, characterization and in vitro antitumour activity of di-n-butyl, tri-n-butyl and triphenyltin 3,6-dioxaheptanoates and 3,6,9-trioxadecanoates. Metal-Based Drugs 5:189–196. doi:10.1155/MBD.1998.189
Kemmer M, Ghys L, Gielen M, Biesemans M, Tiekink ERT, Willem R (1999) Synthesis and characterization of triphenyl-, tri-n-butyl and di-n-butyltin derivatives of 4-carboxybenzo-18-crown-6 and -15-crown-5. J Organomet Chem 582:195–203. doi:10.1016/S0022-328X(99)00035-2
Gielen M, Lelieveld P, de Vos D, Pan H, Willem R, Biesemans M, Fiebig HH (1992) In vitro effect of organotin-substituted steroids in human tumor cell lines. Inorg Chim Acta 196:115–117. doi:10.1016/S0020-1693(00)82967-9
Gielen M, Willem R, Dalil H, de Vos D, Kuiper CM, Peters GJ (1998) Toxicity profiles in vivo in mice and antitumour activity in tumour-bearing mice of di- and triorganotin compounds. Metal-Based Drugs 5:83–90. doi:10.1155/MBD.1998.83
Tiekink ERT (1994) The rich diversity in tin carboxylate structures. Trends Organomet Chem 1:71–116
Dakternieks D, Basu Baul TS, Dutta S, Tiekink ERT (1998) Synthesis, characterization, and X-ray structures of diphenyltin(IV) N-(2-hydroxyacetophenone)glycinate, its 1:1 adduct with triphenyltin(IV) chloride, and related systems. Organometallics 17:3058–3062. doi:10.1021/om9800290
Basu Baul TS, Dutta S, Tiekink ERT (1999) Crystal structure of diphenyltin(IV) N-(2-hydroxy-5-methylacetophenone)glycinate, C23H21NO3Sn. Z Kristallogr 214:361–362
Basu Baul TS, Dutta S, Rivarola E, Choudhuri S (2001) Synthesis, characterization of diorganotin(IV) complexes of N-(2-hydroxyarylidine)aminoacetic acid and antitumour screening in vivo in Ehrlich ascites carcinoma cells. Appl Organomet Chem 15:947–953. doi:10.1002/aoc.245
Basu Baul TS, Dutta S, Rivarola E, Butcher R, Smith FE (2002) The synthesis and structural characterization of some triorganotin(IV) complexes of 2-{[(E)-1-(2-hydroxyaryl)alkylidene]amino}acetic acid. Crystal and molecular structures of Ph3Sn(2-OHC6H4C(H)=NCH2COO) and Me3Sn(2-OHC6H4C(CH3)=NCH2COO). J Organomet Chem 654:100–108. doi:10.1016/S0022-328X(02)01387-6
Basu Baul TS, Dutta S, Masharing C, Rivarola E, Englert U (2003) Organotin(IV) complexes of N-[(2Z)-3-hydroxy-1-methyl-2-butenylidene]glycine. Heteroatom Chem 14:149–154. doi:10.1002/hc.10116
Yin H, Wang Q, Xue S (2004) Synthesis and structural characterization of diorganotin(IV) esters of salicylidene-amino acids. J Organomet Chem 689:2480–2485. doi:10.1016/j.jorganchem.2004.05.004
Basu Baul TS, Masharing C, Willem R, Bieseman M, Holčapek M, Jirásko R, Linden A (2005) Self-assembly of diorganotin(IV) 2-{[(E)-1-(2-oxyaryl)alkylidene]amino}acetates: An investigation of structures by X-ray diffraction, solution and solid state tin NMR, and electrospray ionisation MS. J Organomet Chem 690:3080–3094. doi:10.1016/j.jorganchem.2005.03.010
Linden A, Basu Baul TS, Masharing C (2005) Chloro{μ-2-[(E)-1-(2-oxido-3-methylphenyl) ethylideneamino]acetato}pentaphenylditin(IV). Acta Crystallogr E61:m557–m559
Basu Baul TS, Masharing C, Basu S, Rivarola E, Holčapek M, Jirásko R, Lyčka A, de Vos D, Linden A (2006) Synthesis, characterization, cytotoxic activity and crystal structures of tri- and di-organotin(IV) complexes constructed from the β-{[(E)-1-(2-hydroxyaryl)alkylidene]amino}propionate and β-{[(2Z)-(3-hydroxy-1-methyl-2-butenylidene)]amino}propionate skeletons. J Organomet Chem 691:952–965. doi:10.1016/j.jorganchem.2005.10.057
Basu Baul TS, Masharing C, Rivarola E, Smith FE, Butcher J (2006) Synthesis and characterization of tribenzyltin(IV) and dibenzyltin(IV) complexes of 2-{[(2Z)-3-hydroxy-1-methyl-2-butenylidene]amino}acetic acid. Crystal structure of tribenzyl{2-{[(2Z)-3-hydroxy-1-methyl-2-butenylidene]amino}acetate}tin(IV). Struct Chem 18:231–235. doi:10.1007/s11224-006-9085-2
Basu Baul TS, Masharing C, Ruisi G, Jirásko R, Holčapek M, de Vos D, Wolstenholme D, Linden A (2007) Self-assembly of extended schiff base amino acetate skeletons, 2-{[(2Z)-(3-hydroxy-1-methyl-2-butenylidene)]amino}phenylpropionate and 2-{[(E)-1-(2-hydroxyaryl)alkylidene]amino}phenylpropionate skeletons incorporating organotin(IV) moieties: Synthesis, spectroscopic characterization, crystal structures, and in vitro cytotoxic activity. J Organomet Chem 692:4849–4862. doi:10.1016/j.jorganchem.2007.06.061
Hooft R (1999) KappaCCD Collect Software, Nonius BV, Delft, The Netherlands
Otwinowski Z, Minor W (1997) Processing of X-ray diffraction data collected in oscillation mode. In: Carter Jr CW, Sweet RM (eds) Methods in Enzymology: Macromolecular Crystallography, Part A. vol. 276. Academic, New York, pp 307–326
Blessing RH (1995) An empirical correction for absorption anisotropy. Acta Crystallogr A 51:33–38. doi:10.1107/S0108767394005726
Altomare A, Cascarano G, Giacovazzo C, Guagliardi A, Burla MC, Polidori G, Camalli M (1994) SIR92 - a program for automatic solution of crystal structures by direct methods. J Appl Cryst 27:435. doi:10.1107/S002188989400021X
Beurskens PT, Admiraal G, Beurskens G, Bosman WP, Garcia-Granda S, Gould RO, Smits JMM, Smykalla C (1992) PATTY: The DIRDIF program system, Technical report of the crystallography laboratory. University of Nijmegen, The Netherlands
Beurskens PT, Admiraal G, Beurskens G, Bosman WP, de Gelder R, Israel R, Smits JMM (1994) DIRDIF94: The DIRDIF program system, technical report of the crystallography laboratory. University of Nijmegen, The Netherlands
Flack HD, Bernardinelli G (1999) Absolute structure and absolute configuration. Acta Crystallogr A55:908-915; Flack HD, Bernardinelli G (2000) Reporting and evaluating absolute-structure and absolute-configuration determinations. J Appl Cryst 33:1143–1148. doi:10.1107/S0021889800007184
Sheldrick GM (1997) shelxl97, Program for the refinement of crystal structures. University of Göttingen, Germany
Wang J, Yang X, Cheng J, Xu Y, Liu B, Wang H, Zhang D (1996) Thermodynamic α-CH acidities of amino acid fragments in five co-ordinate bicycyloazastannoxides in Me2SO. J Chem Soc Dalton Trans. 3889–3891. doi:10.1039/dt9960003889
Wang J, Zhang Y, Xu Y, Wang Z (1992) Synthesis and characterization of pentacoordinate organo-tin(IV) complexes. Heteroatom Chem 3:599–602. doi:10.1002/hc.520030523
Matsubayashi G, Tanaka T, Nishigaki S, Nakatsu K (1979) Nuclear magnetic resonance studies of some N′-substituted pyridine-2-carbaldimine adducts of n-butyltrichlorotin(IV) and the molecular structure of n-butyltrichloro(N′-phenylpyridine-2-carbaldimine-NN′)tin(IV). J Chem Soc Dalton Trans. 501–505. doi:10.1039/dt9790000501
van Koten G, Noltes JG (1976) Novel chiral triorganotin halides. Stabilization of optically active tin centers by intramolecular coordination. J Am Chem Soc 98:5393–5395. doi:10.1021/ja00433a058
Holeček J, Nádvorník M, Handliř K, Lyčka A (1983) 13C and 119Sn NMR Study of some four- and five-coordinate triphenyltin(IV) compounds. J Organomet Chem 241:177–184. doi:10.1016/S0022-328X(00)98505-X
Willem R, Verbruggen I, Gielen M, Biesemans M, Mahieu B, Basu Baul TS, Tiekink ERT (1998) Correlating Mössbauer, solution and solid state 117Sn NMR data with X-ray diffraction structural data of triorganotin 2-[(E)-2-(2-hydroxy-5-methylphenyl)-1-diazenyl]benzoates. Organometallics 17:5758–5766. doi:10.1021/om980504u
Boyd MR (1989) Status of the NCI preclinical antitumor drug discovery screen. Principles and practice of oncology, vol 3, pp 1–12. Lippincott, Philadelphia
Keepers YP, Pizao PR, Peters GJ, Ark-Otte JV, Winograd B, Pinedo HM (1991) Comparison of the sulforhodamine B protein and tetrazolium (MTT) assays for in vitro chemosensitivity testing. Eur J Cancer 27:897–900
Nath M, Yadav R, Gielen M, Dalil H, de Vos D, Eng G (1997) Synthesis, characteristic spectral studies and in vitro antimicrobial and antitumour activities of organotin(IV) complexes of Schiff bases derived from amino-acids. Appl Organomet Chem 11:727–736. doi:10.1002/(SICI)1099-0739(199709)11:9<727::AID-AOC639>3.0.CO;2-X
Ogwuru N, Khoo LE, Eng G (1998) A study of the antitumor activity of four dibutyltin(IV)-N-arylidene-α-amino acid complexes. Appl Organomet Chem 12:409–417. doi:10.1002/(SICI)1099-0739(199806)12:6<409::AID-AOC707>3.0.CO;2-F
Tian L, Sun Y, Qian B, Yang G, Yu Y, Shang Z, Zheng X (2005) Synthesis, characterization and biological activity of a novel binuclear organotin complex, Ph3Sn(HL)·Ph2SnL L = 3,5-Br2-2-OC6H2CH=NCH(i-Pr)COO. Appl Organomet Chem 19:1127–1131. doi:10.1002/aoc.968
Tian L, Sun Y, Yang G, Qian B, Shang Z (2005) Formation, structure and in vitro antitumour activity of a novel binuclear organotin complex. Chin Chem Lett 16:1584–1586
Casini A, Messori L, Orioli P, Gielen M, Kemmer M, Willem R (2001) Interactions of two cytotoxic organotin(IV) compounds with calf thymus DNA. J Inorg Biochem 85:297–300. doi:10.1016/S0162-0134(01)00215-X
Acknowledgements
The financial support of the Department of Science & Technology, New Delhi, India (Grant No.SR/S1/IC-03/2005,TSBB) and the University Grants Commission, New Delhi, India through SAP-DSA, Phase-III, are gratefully acknowledged. The in vitro cytotoxicity experiments were carried out by Ms. P. F. van Cuijk in the Laboratory of Translational Pharmacology, Department of Medical Oncology, Erasmus Medical Center, Rotterdam, The Netherlands, under the supervision of Dr. E. A. C. Wiemer and Prof. Dr. G. Stoter.
Author information
Authors and Affiliations
Corresponding authors
Appendix
Appendix
Supplementary Material
CCDC-696775 and CCDC-696776 contain the supplementary crystallographic data for complexes 1 and 3, respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Rights and permissions
About this article
Cite this article
Basu Baul, T.S., Basu, S., de Vos, D. et al. Amino acetate functionalized Schiff base organotin(IV) complexes as anticancer drugs: synthesis, structural characterization, and in vitro cytotoxicity studies. Invest New Drugs 27, 419–431 (2009). https://doi.org/10.1007/s10637-008-9189-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-008-9189-1