Abstract
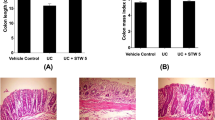
The dextran sulfate sodium (DSS) colitis model has been utilized to screen for novel therapeutics for ulcerative colitis. Evidence suggests the small intestine may also be affected by DSS. We characterized the effects of DSS on the small intestine and assessed the potential for Lactobacillus fermentum BR11 to modify or normalize DSS-induced changes. Rats were allocated to three groups, Water + Vehicle, DSS + Vehicle, and DSS + L. fermentum BR11. BR11 was administered twice daily for 14 days. DSS (2%) was provided from days 7 to 14. Small-intestinal tissue was analyzed for sucrase activity, histology, and crypt cell proliferation. Increased ileum crypt depth and cell proliferation was observed in DSS-treated rats compared to controls (P < 0.05). BR11 normalized these parameters. While DSS predominantly induces colonic damage, minor morphological alterations were also detected in the distal small intestine. L. fermentum BR11 normalized these features.



Similar content being viewed by others
References
Geier MS, Butler RN, Howarth GS. Inflammatory bowel disease: current insights into pathogenesis and new therapeutic options; probiotics, prebiotics and synbiotics. Int J Food Microbiol. 2007;115:1–11. doi:10.1016/j.ijfoodmicro.2006.10.006.
Vowinkel T, Kalogeris TJ, Mori M, Krieglstein CF, Granger DN. Impact of dextran sulfate sodium load on the severity of inflammation in experimental colitis. Dig Dis Sci. 2004;49:556–564. doi:10.1023/B:DDAS.0000026298.72088.f7.
Geier MS, Tenikoff D, Yazbeck R, McCaughan GW, Abbott CA, Howarth GS. Development and resolution of experimental colitis in mice with targeted deletion of dipeptidyl peptidase iv. J Cell Physiol. 2005;204:687–692. doi:10.1002/jcp.20333.
Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002;15:79–94. doi:10.1128/CMR.15.1.79-94.2002.
Tham DM, Whitin JC, Cohen HJ. Increased expression of extracellular glutathione peroxidase in mice with dextran sodium sulfate-induced experimental colitis. Pediatr Res. 2002;51:641–646.
Gaudier E, Michel C, Segain JP, Cherbut C, Hoebler C. The VSL#3 probiotic mixture modifies microflora but does not heal chronic dextran-sodium sulfate-induced colitis or reinforce the mucus barrier in mice. J Nutr. 2005;135:2753–2761.
Gaudio E, Taddei G, Vetuschi A, et al. Dextran sulfate sodium (DSS) colitis in rats: clinical, structural, and ultrastructural aspects. Dig Dis Sci. 1999;44:1458–1475. doi:10.1023/A:1026620322859.
Kim EJ, Chung MY, Chung HJ, et al. Pharmacokinetics of 7-carboxymethyloxy-3′,4′,5-trimethoxy flavone (da-6034), a derivative of flavonoid, in mouse and rat models of chemically induced inflammatory bowel disease. J Pharm Pharmacol. 2006;58:27–35. doi:10.1211/jpp.58.1.0004.
Ohtsuka Y, Sanderson IR. Dextran sulfate sodium-induced inflammation is enhanced by intestinal epithelial cell chemokine expression in mice. Pediatr Res. 2003;53:143–147.
Tanaka T, Kohno H, Suzuki R, et al. Dextran sodium sulfate strongly promotes colorectal carcinogenesis in apc(min/+) mice: Inflammatory stimuli by dextran sodium sulfate results in development of multiple colonic neoplasms. Int J Cancer. 2006;118:25–34. doi:10.1002/ijc.21282.
Takahashi S, Kawamura T, Kanda Y, et al. Multipotential acceptance of Peyer’s patches in the intestine for both thymus-derived t cells and extrathymic t cells in mice. Immunol Cell Biol. 2005;83:504–510. doi:10.1111/j.1440-1711.2005.01361.x.
Grabig A, Paclik D, Guzy C, et al. Escherichia coli strain Nissle 1917 ameliorates experimental colitis via toll-like receptor 2- and toll-like receptor 4-dependent pathways. Infect Immun. 2006;74:4075–4082. doi:10.1128/IAI.01449-05.
Kruis W, Fric P, Pokrotnieks J, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi:10.1136/gut.2003.037747.
Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107–1114. doi:10.1016/S0016-5085(99)70013-2.
Dieleman LA, Goerres MS, Arends A, et al. Lactobacillus gg prevents recurrence of colitis in HLA-b27 transgenic rats after antibiotic treatment. Gut. 2003;52:370–376. doi:10.1136/gut.52.3.370.
Setoyama H, Imaoka A, Ishikawa H, Umesaki Y. Prevention of gut inflammation by bifidobacterium in dextran sulfate-treated gnotobiotic mice associated with bacteroides strains isolated from ulcerative colitis patients. Microbes Infect. 2003;5:115–122. doi:10.1016/S1286-4579(02)00080-1.
Shibolet O, Karmeli F, Eliakim R, et al. Variable response to probiotics in two models of experimental colitis in rats. Inflamm Bowel Dis. 2002;8:399–406. doi:10.1097/00054725-200211000-00004.
Bibiloni R, Fedorak RN, Tannock GW, et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539–1546. doi:10.1111/j.1572-0241.2005.41794.x.
Herias MV, Koninkx JF, Vos JG, Huisin’t Veld JH, van Dijk JE. Probiotic effects of Lactobacillus casei on DSS-induced ulcerative colitis in mice. Int J Food Microbiol. 2005;103:143–155. doi:10.1016/j.ijfoodmicro.2004.11.032.
Osman N, Adawi D, Ahrne S, Jeppsson B, Molin G. Modulation of the effect of dextran sulfate sodium-induced acute colitis by the administration of different probiotic strains of lactobacillus and bifidobacterium. Dig Dis Sci. 2004;49:320–327. doi:10.1023/B:DDAS.0000017459.59088.43.
Rachmilewitz D, Katakura K, Karmeli F, et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–528. doi:10.1053/j.gastro.2003.11.019.
Moon G, Myung SJ, Jeong JY, et al. Prophylactic effect of Lactobacillus GG in animal colitis and its effect on cytokine secretion and mucin gene expressions. Korean J Gastroenterol. 2004;43:234–245.
Geier MS, Butler RN, Giffard PM, Howarth GS. Lactobacillus fermentum BR11, a potential new probiotic, alleviates symptoms of colitis induced by dextran sulfate sodium (DSS) in rats. Int J Food Microbiol. 2007;114:267–274. doi:10.1016/j.ijfoodmicro.2006.09.018.
Tomas FM, Knowles SE, Owens PC, et al. Effects of full-length and truncated insulin-like growth factor-i on nitrogen balance and muscle protein metabolism in nitrogen-restricted rats. J Endocrinol. 1991;128:97–105.
Pelton NC, Tivey DR, Howarth GS, Davidson GP, Butler RN. A novel breath test for the non-invasive assessment of small intestinal mucosal injury following methotrexate administration in the rat. Scand J Gastroenterol. 2004;39:1015–1016. doi:10.1080/00365520410003416.
Tooley KL, Howarth GS, Lymn KA, Lawrence A, Butler RN. Oral ingestion of streptococcus thermophilus diminishes severity of small intestinal mucositis in methotrexate treated rats. Cancer Biol Ther. 2006;5:593–600. doi:10.1158/1535-7163.MCT-05-0457.
Mauger CA, Butler RN, Geier MS, Tooley KL, Howarth GS. Probiotic effects on 5-fluorouracil-induced mucositis assessed by the sucrose breath test in rats. Dig Dis Sci. 2007;52:612–619. doi:10.1007/s10620-006-9464-y.
Torres DM, Tooley KL, Butler RN, Smith CL, Geier MS, Howarth GS. Lyprinol only partially improves indicators of small intestinal integrity in a rat model of 5-fluorouracil-induced mucositis. Cancer Biol Ther. 2008;7:295–302.
Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968;22:99–107. doi:10.1016/0003-2697(68)90263-7.
Clarke JM, Pelton NC, Bajka BH, Howarth GS, Read LC, Butler RN. Use of the C-13-sucrose breath test to assess chemotherapy-induced small intestinal mucositis in the rat. Cancer Biol Ther. 2006;5:34–38.
Hong MY, Turner ND, Carroll RJ, Chapkin RS, Lupton JR. Differential response to DNA damage may explain different cancer susceptibility between small and large intestine. Exp Biol Med (Maywood). 2005;230:464–471.
Howarth GS, Tooley KL, Davidson GP, Butler RN. A non-invasive method for detection of intestinal mucositis induced by different classes of chemotherapy drugs in the rat. Cancer Biol Ther. 2006;5:1189–1195.
Acknowledgments
The authors would like to acknowledge Kerry Lymn, Roger Yazbeck, Lynn Scarman, and Katie Tooley for assistance with animal trials. Jane Chapman and Kelly Roberts assisted with image analysis. The authors would also like to acknowledge Betty Zacharakis and Esther Burt for analysis of breath samples by isotope ratio mass spectrometer. Associate Professor Gordon Howarth is supported by a Cancer Council South Australia Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geier, M.S., Smith, C.L., Butler, R.N. et al. Small-Intestinal Manifestations of Dextran Sulfate Sodium Consumption in Rats and Assessment of the Effects of Lactobacillus fermentum BR11. Dig Dis Sci 54, 1222–1228 (2009). https://doi.org/10.1007/s10620-008-0495-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-008-0495-4