Abstract
Background
The standardized herbal preparation, STW 5, is effective clinically in functional gastrointestinal disorders and experimentally in ulcerative colitis (UC). The present study explores whether the beneficial effect of STW 5 involves influencing the intestinal microbiota.
Methods
UC was induced in Wistar rats by feeding them 5% dextran sodium sulfate (DSS) in drinking water for 7 days. Rats were treated concurrently with STW 5 and sacrificed 24 h after last drug administration. Fecal samples were used to determine changes in the abundance of selected microbial phyla and genera using real-time PCR.
Results
Induction of UC led to dysbiosis and changes in the gut microbiota. The changes included an increase in some genera of the Firmicutes, namely Enterococcus, and a decrease in others, namely Blautia, Clostridium, and Lactobacillus. DSS further induced a marked increase in the abundance of Bacteroidetes and Proteobacteria as well as in the relative abundance of Actinobacteria and its genus Bifidobacterium. Methanobrevibacter levels (phylum Euryarchaeota) were also increased. Microbial dysbiosis was associated with changes in various parameters of colonic inflammation. STW 5 effectively guarded against those changes and significantly affected the indices of edema and inflammation in the UC model. Changes in colon length, colon mass index, inflammatory and apoptotic markers, and histological changes induced by DSS were also prevented.
Conclusions
Dysbiosis plays a contributing role in the development of DSS-induced UC. Derangements in the microbial flora and associated inflammatory processes were largely prevented by STW 5, suggesting that this effect might contribute towards its beneficial usefulness in this condition.
Similar content being viewed by others
Background
Gastrointestinal diseases (GID), whether inflammatory or functional (FGID), affect people worldwide and impair their quality of life and work productivity [1]. Inflammatory bowel diseases (IBD) are usually manifested either as ulcerative colitis (UC) or as Crohn’s disease (CD) [2]. Colitis induced by dextran sodium sulfate (DSS) in rats mimics the clinical and histological features of UC by interfering with intestinal barrier function and stimulating local inflammatory processes [3]. Growing evidence further suggests the involvement of gut microbiota in IBD [4]. It has been postulated that the gut microbiota imbalance (dysbiosis) could initiate immune responses by compromising the mucosal barrier and stimulating local and systemic immunity [5, 6]. This fact qualifies the DSS model to be used as a dysbiosis model [7].
Furthermore, altered motility, visceral hypersensitivity, immune alterations, low-grade inflammation, dysfunctional brain-gut axis, and compromised epithelial barrier function have all been postulated to contribute to the symptoms in functional dyspepsia (FD) and irritable bowel syndrome (IBS) [8, 9]. Gut microbiota has been shown to modulate many of these physiological functions [10, 11]. Although no consistent microbial signature has been associated with FGIDs, several lines of evidence support a role for gut microbes in the development of FGID symptoms [11].
Gut microbiota is a complex ecosystem dominated by four main phyla: Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria [12, 13]. In a healthy state, the gut microbiota has a mutualistic relationship with the human host. The host intestine provides the microbes with a niche and the microbial ecosystem contributes to maintaining homeostasis by modulating several physiological functions such as nutrient digestion, immune responses, and normal perception of visceral pain [14].
STW 5 (Iberogast®) is a standardized multi-component herbal preparation consisting of a combination of nine medicinal herbal extracts, commercially available in Europe. It was shown to be effective in FD and IBS in several randomized clinical studies [15] and was previously reported to have anti-ulcerogenic and mucosal protective effects as well as potent anti-inflammatory properties [16]. The present study aimed at exploring whether the beneficial effect of STW 5 could also involve modulation of the intestinal microbiota.
Methods
Animals
Adult female Wistar rats, weighing 150–200 g each, were obtained from the Modern Veterinary Office for Laboratory Animals, Cairo, Egypt. Rats were provided with a standard pellet diet and were given water ad libitum. The animals were housed at a temperature of 22 ± 3 °C and a 12-h light/dark cycle as well as at a constant relative humidity throughout the experimental period. Animals were left to acclimatize for at least 7 days before subjecting them to experimentation.
The study was carried out in compliance with the ARRIVE guidelines and experimental procedures were approved by the institutional Ethical Committee for Animal Experimentation at the Faculty of Pharmacy, Cairo University, Cairo, Egypt, approval number (PT 1769) following the guidelines laid out in the Guide for the Care and Use of Laboratory Animals, National Academy of Science.
Drugs
STW5 (Iberogast®) is a commercially available standardized herbal preparation that was generously provided by Bayer consumer health (Darmstadt, Germany). It consists of hydroethanolic extracts of Iberis amara L. (Brassicaceae) (15%), Melissa officinalis L. (Lamiaceae) (10%), Matricaria chamomilla (Compositae) (20%), Carum carvi L. (Apiaceae) (10%), Mentha piperita L. (Lamiaceae) (5%), Angelica archangelica L. (Apiaceae) (10%), Silybum marianum (L.) Gaertn. (Compositae) (10%), Chelidonium majus L. (Papaveraceae) (10%), and Glycyrrhiza glabra L. (Leguminosae) (10%). The preparation and every single extract were well characterized according to the guidelines of the European Medicines Agency. The extraction processes as well as the quality controls were previously described in detail [17]. Briefly, the extracts were prepared, and quality controlled according to Good Manufacturing Practice and Good Agricultural Practice of Medicinal and Aromatic Plants. The quality of each extract was tested according to individual specifications as chromatographic fingerprint [17, 18].
Induction of colitis
Colitis was induced in rats by adding DSS, molecular weight 37–40 kD, (TdB Consultancy, Uppsala, Sweden), to the drinking water in a concentration of 5% (w/v) for 1 week [19].
Experimental design
Adult rats were randomly allocated to three groups of 14–16 animals each as follows:
-
(a)
Vehicle control group: received normal tap water (without DSS) and given 31% ethanol (STW 5 vehicle) 5 mL/kg, orally daily for 1 week.
-
(b)
UC group: received 5% DSS in drinking water and given concurrently 31% ethanol (STW 5 vehicle) 5 mL/kg, orally daily for 1 week.
-
(c)
UC/STW 5 group: received 5% DSS in drinking water and given concomitantly STW 5 (5 mL/kg), orally daily for 1 week. This dose was chosen after carrying out preliminary experiments with two doses (2 ml and 5 ml /kg) of the preparation and selected on the basis that it had more consistent effects on the intestinal microbiota.
Twenty-four hours after the last drug administration, rats were euthanized using halothane anesthesia followed by cervical dislocation and the colon and caecum from all animals were excised. The colon length was measured, rinsed in ice-cold saline, cleaned of extraneous tissue, dried on filter paper, and weighed. The ratio of colon weight in milligrams to the total body weight in grams was taken as the colon mass index and was used as a measure of the degree of colonic edema and severity of inflammation. The colon was then cut longitudinally into two segments: one was fixed in 10% formalin for histological examination, and the other was homogenized in ice-cold saline to obtain a 10% homogenate for the assessment of biochemical parameters. The entire caeca were stored at -20 °C until further use. Fecal samples from each caecum were used for microbial genomic DNA isolation and further analysis.
Determination of biochemical parameters associated with colitis
The colon homogenate was centrifuged at 6000 rpm for 30 min at 4 °C. The supernatant was used for assaying tumor necrosis factor-alpha (TNF-α), nuclear factor kappa B (NFκB), and caspase-3 using rat specific enzyme-linked immunosorbent assay (ELISA) kits from Elabscience Biotechnology Co. (Texas, USA), Hangzhou Eastbiopharm Co. (Hangzhou, China) and Cloud-Clone corp. (Texas, USA), respectively.
Microbial genomic DNA isolation
The excised frozen caeca were subjected to fecal genomic DNA extraction using Zymo research fecal DNA extraction kit (Zymo Research Corp., Irvine, CA, USA) following the manufacturer’s protocol. This protocol was effective in removing traces of DSS from the isolated DNA, as DSS is a PCR inhibitor [20]. DNA concentration was determined by measuring the absorbance at 260 nm using Implen nanophotometer P-330 (Implen GmbH, Munich, Germany).
Relative abundance of microbial phyla using quantitative real-time PCR
The changes in the main gut-associated microbiota were quantified using specific primers targeting different microbial genera 16S ribosomal ribonucleic acid (rRNA) gene by Real Time-PCR (qPCR) as described in Table 1. qPCR experiments were performed using QuantiFast SYBR Green PCR Kit (Qiagen, Hilden, Germany) on a Rotor-Gene Real-Time PCR machine (Qiagen, Hilden, Germany). The thermal cycling conditions were optimized as follows: an initial DNA denaturation step at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 10 s, primer annealing at optimal temperature for 20 s, extension at 72 °C for 15 s. Melt curve analysis was performed by slowly cooling from 95 °C to 60 °C (0.05 °C per cycle) with simultaneous measurement of the SYBR Green I signal intensity. All PCR tests were carried out in duplicates of each group pool.
Microbial genomic DNA standard curves
Microbial genomic DNAs used for the construction of the standard curves were extracted from Lactobacillus acidophilus (ATCC 4356) and Enterococcus faecalis (ATCC 19433) obtained from the American type culture collection (Virginia, USA). The genomic DNA of Escherichia coli (DSM 498), Prevotella intermedia (DSM 20706), Blautia producta (DSM 2950), Bacteroides vulgatus (DSM 1447), Methanobrevibacter smithii (DSM 861), Ilyobacter polytropus (DSM 2926), Clostridium leptum (DSM 753) and Bifidobacterium bifidum (DSM 20456) were obtained from Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany. Standard curves were constructed for each experiment using serial ten-fold dilution of microbial genomic DNA of the mentioned standard cultures of microbes, corresponding to 30 to 3 × 106 16S rRNA gene copies. The mass for one microbial genome was calculated by using Avogadro’s number and assuming the mean molecular weight of a base pair to be 660 g/mol. Standard curves were normalized to the copy number of the 16S rRNA gene for each microbial species. The microbial concentration from each fecal sample was calculated by comparing the cycle threshold (Ct) values obtained from the standard curves and expressed as gene copies/ gram of feces.
Histopathological examination of the colon
Transverse sections, 4–6 μm thin, were prepared from paraffin-embedded colon segments from each animal of all experimental groups. The sections were stained with hematoxylin and eosin (H&E) and examined under 200 magnification using a light microscope by a pathologist blinded to the treatment regimens.
Statistical analysis
All data obtained were presented as means ± SEM. Results were analyzed using a one-way analysis of variance test (one-way ANOVA) followed by Tukey’s multiple comparison test. Statistical analysis was performed using Graph Pad Prism software (version 6.04). For all the statistical tests, the level of significance was taken at p < 0.05.
Results
Effect on colon length, colon mass index, colon histology, inflammatory and apoptotic biomarkers
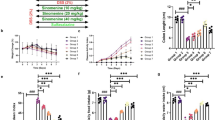
Induction of colitis led to a reduction in rat colon length by approximately 20% (Fig. 1a). This was associated with an 18% increase in colon mass index (Fig. 1b). Histological photomicrographs of control colons showed well-defined crypt lengths and no edema in the mucosa and submucosa (Fig. 1c). However, DSS-treated animals (Fig. 1d) showed loss of epithelial cell and crypt architecture, inflammatory cell infiltration with marked necrosis of the epithelium, and submucosal edema. Treatment with STW 5 (Fig. 1e) largely protected against these histological changes. Furthermore, DSS induced changes in various parameters indicative of inflammation and apoptosis as evidenced by a marked elevation in the colonic content of TNF-α, NFκB (Fig. 2 a and b), and caspase-3 level (Fig. 2 c). These changes tended to be prevented by treatment with STW 5.
Effect of STW 5 treatment on colon length, colon mass index, and histopathological changes, in colonic tissue of rats with DSS-induced colitis. Induction of colitis led to a reduction in colon length A as compared to the vehicle control group and this was associated with an increase in colon mass index B. Normal histological structure of colonic mucosa in normal control rats C. Colon of rats with DSS-induced colitis showing necrosis of epithelium, distortion of crypts, inflammatory infiltrate in lamina propria as well as sub-mucosal edema D. Apparently normal mucosa in the colon of rats with DSS-induced colitis after treatmet with STW 5 which tended to prevent these changes E. Data represented as means ± standard deviation of at least two independent experiments with number of animals of at least 14 animals per group
Effect of STW 5 treatment on inflammatory and apoptotic biomarkers in colonic tissue of rats with DSS induced UC. TNF-α, A, NFκB B, and caspase-3 C measurd by ELISA were significantly elevated in DSS-induced colitis, but this rise was prevented by STW 5 treatment. Data represented as means ± standard deviation of at least two independent experiments with number of animals of 6 animals per group
Effect on intestinal microbiota
DSS administration led to a 15-fold increase in the Enterococcus population of the Firmicutes phylum whereas the other three representatives of the phylum, namely, Clostridium, Lactobacillus, and Blautia, showed a decrease by 23, 73, and 28% respectively (Fig. 3). All the studied representative members of the Bacteriodetes phylum showed an increase in the UC model, an effect that was largely prevented by the herbal preparation STW 5 except for Bacteroides (Fig. 4). Furthermore, DSS led to a nearly 3-fold increase in the Actinobacteria phylum (Fig. 5), but a 500-fold increase in Bifidobacterium (Fig. 5). DSS administration led to a 20-fold increase in the relative abundance of Proteobacteria, an effect which was significantly reduced to only 3% after STW 5 treatment (Fig. 6). Changes in 16S rRNA DNA showed an increased level of Methanobrevibacter by 2.5-fold (Fig. 7 a) while levels of Fusobacterium phylum were not significantly affected by DSS (Fig. 7 b). STW 5 treatment guarded against all the changes induced by DSS.
Effect of STW 5 treatment on the relative abundace of Firmicutes phylum, Enterococcus A, Clostridium B, Lactobacillus C, Balutia D in rats with DSS induced UC. Members of Firmicutes phylum were affected differently. Enterococcus population showed 15-fold increase as compared to vehicle control group and STW 5 treatment completely normalized its level. On the other hand, Clostridium, Lactobacillus and Blautia decreased by 1.3, 3.7 and 1.4 folds (23, 73 and 28%), respectively. STW 5 tended to slightly increase these levels. The microbial concentration expressed as ng per gram of feces was calculated taking 16S rRNA gene copy number into consideration as detailed in the Methods section. Data represented as means ± standard deviation of at least two independent experiments with number of animals of at least 14 animals per group
Effect of STW 5 treatment on the relative abundance of Bacteriodetes phylum A, Bacteroides B, Prevotella C in rats with DSS induced UC. All members of Bacteriodetes phylum showed a significant increase in DSS-induced colitis model. STW 5 succeeded to significantly reverse these changes. The microbial concentration expressed as ng per gram of feces was calculated taking 16S rRNA gene copy number into consideration as detailed in methods section. Data represented as means ± standard deviation of at least two independent experiments with number of animals of at least 14 animals per group
Effect of STW 5 treatment on the relative abundance of Actinobacteria A and Bifidobacterium B in rats with DSS induced UC. Actinobacteria and Bifidobacterium displayed the same pattern of change. DSS induced colitis led to a 2.8 and 486-fold increase in Actinobacteria and Bifidobacterium respectively, an effect which was significantly reversed by STW 5 treatment. The microbial concentration expressed as ng per gram of feces was calculated taking 16S rRNA gene copy number into consideration as detailed in the Methods section. Data represented as means ± standard deviation of at least two independent experiments with number of animals of at least 14 animals per group
Effect of STW 5 treatment on the relative abundance of Proteobacteria in rats with DSS induced UC. DSS administration led to a 20-fold increase in Proteobacteria relative abundance which was significantly reduced after STW 5 treatment. The microbial concentration expressed as ng per gram of feces was calculated taking 16S rRNA gene copy number into consideration as detailed in the Methods section. Data represented as means ± standard deviation of at least two independent experiments with number of animals of at least 14 animals per group
Effect of STW 5 treatment on relative abundance of Fusobacterium and Methanobrevibacter in rats with DSS induced UC. DSS administration increased Methanobrevibacter A by approximately 2.5 folds compared to vehicle control group. STW 5 succeeded to restore Methanobrevibacter to its normal levels. Meanwhile, DSS slightly decreased Fusobacterium B population and increased levels were caused by STW 5 administration. The microbial concentration expressed as ng per gram of feces was calculated taking 16S rRNA gene copy number into consideration as detailed in the Methods section. Data represented as means ± standard deviation of at least two independent experiments with number of animals of at least 14 animals per group
To gain more insight into the beneficial effects of STW 5 in the DSS model of colitis, it was necessary to show whether the herbal preparation has any effect on the normal microbiota flora or not on its own. The dose of STW 5 (5 ml/kg) have been tested on the microbiota flora in normal rats and was found to be largely insignificant (Supplementary Data).
Relative abundance of bacterial and archaeal phyla
To better understand how STW 5 affects the unbalanced microbial community induced by DSS colitis, the relative abundance of measured phyla and genera was analyzed as shown in Fig. 8. DSS-induced dysbiosis was evident by changes in the relative abundance of Firmicutes, Fusobacterium and Methanobrevibacter phyla and Lactobacillus, Blautia, and Bacteroides genera. Most of these changes have been resolved by STW 5 treatment, particularly those of Blautia and Methanobrevibacter.
Relative proportions of selected phyla and genera of gut microbiota in vehicle control A, UC B and UC + STW 5 C. The relative proportion of Firmicutes as a phylum was increased in UC this was decreased in STW 5 treated group. Within Firmicutes, Lactobacillus population showed about 4-fold decrease in UC, STW 5 tended to slightly increase its levels. DSS administration slightly decreased Fusobacterium population and increased levels were caused by STW 5 administration. The changes in the remaining phyla and genera of gut microbiota represented less than 0.1% of the total proportional changes observed
Discussion
Gut microbiota is known to maintain a balance between its members to preserve intestinal integrity by preventing pathogen colonization and initiation of inflammation. Derangement of this balance is associated with the development of ulcerative colitis in man [29]. Furthermore, alterations in function and relative abundance of intestinal bacteria belonging to the phyla Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria phyla have been implicated in experimental colitis [30]. As well as changes in Fusobacteria and members of Methanobrevibacter genus [31,32,33].
In the present study, DSS-induced colitis was associated with variable changes in the different phyla and genera examined. The genera belonging to the Firmicutes showed a five-fold increase in the relative abundance of Enterococcus but a significant decrease in that of some commensal microbiota including Clostridium cluster IV, Blautia, and Lactobacilli. These species owe their commensality to the fact that they produce short-chain fatty acids (acetic, butyric, and lactic acids) as fermentation end-products that have an essential role in the metabolic welfare of colonocytes [34,35,36]. A decrease in Lactobacillus has been reported in colonic biopsies from patients with active UC [37, 38].
DSS also induced a dramatic increase in Prevotella (phylum Bacteroidetes), conforming with earlier reports [39]. Another relevant bacterial phylum in IBD is Actinobacteria with its genus Bifidobacteria. In the present study, Actinobacteria and Bifidobacterium levels were dramatically increased by DSS similar to previous studies showing increased proportions of Bifidobacterium in UC patients [40]. Furthermore, earlier studies showed an increase in the levels of Poteobacteria in DSS-induced colitis [7] as well as in Fusobacterium varium in the colonic mucosa of UC patients [31]. Our experimental findings showed similar effects regarding Proteobacteria but failed to show significant changes in the Fusobacterium population.
DSS further raised the relative abundance of Methanobrevibacter (phylum Euryarchaeota) in accordance with reports that its levels are increased in UC patients and responsible for the bloating and decreased intestinal motility symptoms [41, 42]. STW 5 tended to normalize Methanobrevibacter abundance, a fact that might explain the clinically proven efficacy of STW 5 in bloating.
Gut microbiota has been shown to play an important role in intestinal inflammatory conditions, some in initiation and progression of the inflammatory process and some in having an anti-inflammatory effect. For example, Proteobacteria has been associated with inflammation in different models of colitis [43, 44] while Bacteroides vulgatus has been shown to activate the signaling of NFκB in the gut epithelial cell culture [45]. However, butyrate which is produced by commensal Clostridia inhibits NF-kβ activation in gut cells leading to an intestinal anti-inflammatory effect [35]. Furthermore, various strains of Bifidobacteria have been shown to exert an anti-inflammatory effect through induction of intestinal IL-10 [40] and treatment with Bifidobacterium bifidum was shown to partially protect mice from Th1-driven inflammation in a chemically induced model of colitis [46]. The present findings show indeed that DSS-induced colitis was associated with a marked increase in inflammatory and apoptotic markers such as TNF-α, NFκB, and caspase-3.
STW 5 administration significantly decreased colon inflammation and apoptosis. The anti-inflammatory effect of STW 5 might be attributed in part to decreasing Proteobacteria and Enterococcus levels and increasing Clostridia population, a fact that might help to explain the reduced inflammation and maintenance of the normal bacterial ecosystem. While the administration of STW 5 itself in normal rats had largely insignificant effects on the tested microbiota (Supplementary Data), yet when given to animals with DSS-induced colitis, it significantly decreased the relative abundance of Bacteroidetes and Prevotella and tended to normalize the abundance of both Actinobacteria and Bifidobacterium populations. Furthermore, STW 5 increased the abundance of Bacteroides, Lactobacillus, Clostridium, Blautia as well as Fusobacterium and succeeded to normalize Methanobrevibacter abundance, a fact that might explain the clinically proven efficacy of STW 5 in bloating.. It is difficult to ascribe the beneficial effect of STW 5 to any one or more of its active constituents. Earlier studies on its gastroprotective effects showed that each individual component contributes to the overall efficacy, but optimal activity was exerted by their combined effects [47, 48]. The preparation as a whole was also shown to be effective in experimental models of functional dyspepsia [49] and colitis [19] as well as clinically in IBS and FD [15, 16]. It would therefore be reasonable to assume that the effects obtained in the present study are the result of the combined activity of the individual components of the standardized preparation.
Conclusion
DSS-induced colitis was associated with gut microbial dysbiosis, an effect that tends to create a pro-inflammatory milieu, initiating intestinal inflammation. This shift in gut microbial composition included reduced beneficial indigenous microbiota that acts to maintain epithelial health. Treatment with STW 5 showed anti-inflammatory and antiapoptotic effects and favorably affected the intestinal microbiota by decreasing bacteria that contribute to intestinal inflammation as Proteobacteria and Prevotella and increasing bacteria with anti-inflammatory properties as Bifidobacteria and Lactobacilli. The results provide an additional novel mechanism of action underlying the beneficial effect of using STW 5 in gastrointestinal disorders.
Availability of data and materials
The raw data generated during the current study are available from the corresponding author upon request.
Abbreviations
- CD:
-
Crohn’s disease
- DSS:
-
Dextran sodium sulfate
- FD:
-
Functional dyspepsia
- FGID:
-
Functional gastrointestinal diseases
- GID:
-
Gastrointestinal diseases
- IBD:
-
Inflammatory bowel diseases
- IBS:
-
Irritable bowel syndrome
- NFκB:
-
Nuclear factor kappa B
- TNF-α:
-
Tumor necrosis factor-alpha
- UC:
-
Ulcerative colitis
References
M'koma AE. Inflammatory bowel disease: an expanding global health problem. Clin Med Insights Gastroenterol. 2013;6:33–47. https://doi.org/10.4137/CGast.S12731.
Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20(1):91–9. https://doi.org/10.3748/wjg.v20.i1.91.
H. Laroui, S. A. Ingersoll, H. C. Liu, , M. T. Baker, S. Ayyadurai, M. A. Charania, F. Laroui, Y. Yan, S.V. Sitaraman, D. Merlin, Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon, PLoS One (2012), 7, e32084, 3, doi: https://doi.org/10.1371/journal.pone.0032084.
Cammarota G, Ianiro G, Cianci R, Bibbò S, Gasbarrini A, Currò D. The involvement of gut microbiota in inflammatory bowel disease pathogenesis: potential for therapy. Pharmacol.Ther. 2015;149:191–212. https://doi.org/10.1016/j.pharmthera.2014.12.006.
Du Z, Hudcovic T, Mrazek J, Kozakova H, Srutkova D, Schwarzer M, et al. Development of gut inflammation in mice colonized with mucosa-associated bacteria from patients with ulcerative colitis. Gut Pathog. 2015;7(1):32. https://doi.org/10.1186/s13099-015-0080-2.
Tannock G. Molecular analysis of the intestinal microflora in IBD. Mucosal Immunol. 2008;1(S1):S15–8. https://doi.org/10.1038/mi.2008.54.
Munyaka PM, Rabbi MF, Khafipour E, Ghia JE. Acute dextran sulfate sodium (DSS)-induced colitis promotes gut microbial dysbiosis in mice. J Basic Microbiol. 2016;56(9):986–98. https://doi.org/10.1002/jobm.201500726.
Stanghellini V, Chan KL, Hasler WL, Malagelada JR, Suzuki H, Tack J, et al. Gastroduodenal Disorders. Gastroenterology. 2016;150(6):1380–92. https://doi.org/10.1053/j.gastro.2016.02.011.
Quigley EM. Bugs on the brain; brain in the gut--seeking explanations for common gastrointestinal symptoms. Ir J Med Sci. 2013;182:1–6.
Shin A, Preidis GA, Shulman R, Kashyap PC. The gut microbiome in adult and pediatric functional gastrointestinal disorders. Clin Gastroenterol Hepatol. 2019;17:256–74.
Tap J, Derrien M, Törnblom H, Brazeilles R, Cools-Portier S, Doré J, et al. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology. 2017;152(1):111–23. https://doi.org/10.1053/j.gastro.2016.09.049.
Hold GL, Pryde SE, Russell VJ, Furrie E, Flint HJ. Assessment of microbial diversity in human colonic samples by 16S rDNA sequence analysis. FEMS Microbiol Ecol. 2002;39(1):33–9. https://doi.org/10.1111/j.1574-6941.2002.tb00904.x.
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. https://doi.org/10.1038/nature11234.
Collins SM. A role for the gut microbiota in IBS. Nat RevGastroenterol Hepatol. 2014;11(8):497–505. https://doi.org/10.1038/nrgastro.2014.40.
Allescher HD, Wagner H. STW 5/Iberogast: multi-target-action for treatment of functional dyspepsia and irritable bowel syndrome. Wien. Med. Wochenschr. 2007;157(13-14):301–7. https://doi.org/10.1007/s10354-007-0429-3.
Ottillinger B, Storr M, Malfertheiner P, Allescher HD. STW 5 (Iberogast®)—a safe and effective standard in the treatment of functional gastrointestinal disorders. Wien Med Wochenschr. 2013;16:65–72.
Kroll U, Cordes C. Pharmaceutical prerequisites for a multi-target therapy. Phytomedicine. 2006;13:12–9. https://doi.org/10.1016/j.phymed.2006.03.016.
Wegener T, Wagner H. The active components and the pharmacological multi-target principle of STW 5 (Iberogast). Phytomedicine. 2006;13:20–35. https://doi.org/10.1016/j.phymed.2006.07.001.
Wadie W, Abdel-Aziz H, Zaki HF, Kelber O, Weiser D, Khayyal MT. STW 5 is effective in dextran sulfate sodium-induced colitis in rats. IntJ Colorectal Dis. 2012;27(11):1445–53. https://doi.org/10.1007/s00384-012-1473-z.
Viennois E, Chen F, Laroui H, Baker MT, Merlin D. Dextran sodium sulfate inhibits the activities of both polymerase and reverse transcriptase: lithium chloride purification, a rapid and efficient technique to purify RNA. BMC ResNotes. 2013;6:360.
Guo X, Xia X, Tang R, Zhou J, Zhao H, Wang K. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett Appl Microbiol. 2008;47(5):367–73. https://doi.org/10.1111/j.1472-765X.2008.02408.x.
Delroisse JM, Boulvin AL, Parmentier I, Dauphin RD, Vandenbol M, Portetelle D. Quantification of Bifidobacterium spp. and Lactobacillus spp. in rat fecal samples by real-time. PCR Res. 2008;163:663–70.
Matsuki T, Watanabe K, Fujimoto J, Takada T, Tanaka R. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Environ Microbiol. 2004;70(12):7220–8. https://doi.org/10.1128/AEM.70.12.7220-7228.2004.
Rinttilä T, Kassinen A, Malinen E, Krogius L, Palva A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol. 2004;97(6):1166–77. https://doi.org/10.1111/j.1365-2672.2004.02409.x.
Bekele AZ, Koike S, Kobayashi Y. Genetic diversity and diet specificity of ruminal Prevotella revealed by 16S rRNA gene-based analysis. FEMS Microbiol Lett. 2010;30:49–57.
Stach JEM, Maldonado LA, Ward AC, Goodfellow M, Bull AT. New primers for the class Actinobacteria: application to marine and terrestrial environments. Environ Microbiol. 2003;5(10):828–41. https://doi.org/10.1046/j.1462-2920.2003.00483.x.
Friswell MK, Gika H, Stratford IJ, Theodoridis G, Telfer B, Wilson ID, et al. Site and strain-specific variation in gut microbiota profiles and metabolism in experimental mice. PLoS One. 2010;5(1):e8584. https://doi.org/10.1371/journal.pone.0008584.
Dridi B, Henry M, El Khéchine A, Raoult D, Drancourt M. High prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol. PLoS One. 2009;4(9):e7063. https://doi.org/10.1371/journal.pone.0007063.
Reinisch W. Fecal microbiota transplantation in inflammatory bowel disease. Dig Dis. 2017;35(1-2):123–6. https://doi.org/10.1159/000449092.
Hansen J, Gulati A, Sartor RB. The role of mucosal immunity and host genetics in defining intestinal commensal bacteria. Curr Opin Gastroenterol. 2010;26(6):564–71. https://doi.org/10.1097/MOG.0b013e32833f1195.
Ohkusa T, Sato N, Ogihara T, Morita K, Ogawa M, Okayasu I. Fusobacterium varium localized in the colonic mucosa of patients with ulcerative colitis stimulates species-specific antibody. J Gastroenterol Hepatol. 2002;17(8):849–53. https://doi.org/10.1046/j.1440-1746.2002.02834.x.
Scanlan PD, Shanahan F, Marchesi JR. Human methanogen diversity and incidence in healthy and diseased colonic groups using mcrA gene analysis. BMC Microbiol. 2008;8(1):79. https://doi.org/10.1186/1471-2180-8-79.
Ghavami SB, Rostami E, Sephay AA, Shahrokh S, Balaii H, Aghdaei HA, et al. Alterations of the human gut Methanobrevibacter smithii as a biomarker for inflammatory bowel diseases. Microb Pathog. 2018;117:285–9. https://doi.org/10.1016/j.micpath.2018.01.029.
Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217(2):133–9. https://doi.org/10.1111/j.1574-6968.2002.tb11467.x.
Segain JP, Raingeard de la Blétiere D, Bourreille A, Leray V, Gervois N, Rosales C, et al. Butyrate inhibits inflammatory responses through NFκB inhibition: implications for Crohn's disease. Gut. 2000;47(3):397–403. https://doi.org/10.1136/gut.47.3.397.
Slover CM, Danziger L. Lactobacillus: a review. Clin Microbiol Newsl. 2008;30(4):23–7. https://doi.org/10.1016/j.clinmicnews.2008.01.006.
Fabia R, Ar’Rajab A, Johansson ML, Andersson R, Willén R, Jeppsson B, et al. Impairment of bacterial flora in human ulcerative colitis and experimental colitis in the rat. Digestion. 1993;54(4):248–55. https://doi.org/10.1159/000201045.
Ott SJ, Plamondon S, Hart A, Begun A, Rehman A, Kamm MA, et al. Dynamics of the mucosa-associated flora in ulcerative colitis patients during remission and clinical relapse. J Clin Microbiol. 2008;46(10):3510–3. https://doi.org/10.1128/JCM.01512-08.
Larsen JM. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151(4):363–74. https://doi.org/10.1111/imm.12760.
Wang W, Chen L, Zhou R, Wang X, Song L, Huang S, et al. Increased proportion of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J Clini Microbiol. 2014;52(2):398–406. https://doi.org/10.1128/JCM.01500-13.
Pimentel M, Lin HC, Enayati P, van den Burg B, Lee HR, Chen JH, et al. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006;290:1089–95.
Kim G, Deepinder F, Morales W, Hwang L, Weitsman S, Chang C, et al. Methanobrevibacter smithii is the predominant methanogen in patients with constipation-predominant IBS and methane on breath. Dig Dis Sci. 2012;57(12):3213–8. https://doi.org/10.1007/s10620-012-2197-1.
Maharshak N, Packey CD, Ellermann M, Manick S, Siddle JP, Huh EY, et al. Altered enteric microbiota ecology in interleukin 10-deficient mice during development and progression of intestinal inflammation. Gut Microbes. 2013;4(4):316–24. https://doi.org/10.4161/gmic.25486.
Selvanantham T, Lin Q, Guo CX, Surendra A, Fieve S, Escalante NK, et al. NKT cell–deficient mice harbor an altered microbiota that fuels intestinal inflammation during chemically induced colitis. J Immunol. 2016;197(11):4464–72. https://doi.org/10.4049/jimmunol.1601410.
Cuiv PE, De Wouters T, Giri R, Mondot S, Smith WJ, Blottiere HM, et al. The gut bacterium and pathobiont Bacteroides vulgatus activates NF-κB in a human gut epithelial cell line in a strain and growth phase dependent manner. Anaerobe. 2017;47:209–17. https://doi.org/10.1016/j.anaerobe.2017.06.002.
Philippe D, Heupel E, Blum-Sperisen S, Riedel CU. Treatment with Bifidobacterium bifidum 17 partially protects mice from Th1-driven inflammation in a chemically induced model of colitis. Int J Food Microbiol. 2011;149(1):45–9. https://doi.org/10.1016/j.ijfoodmicro.2010.12.020.
Khayyal MT, El-Ghazaly MA, Kenawy SA, Seif-el-Nasr M, Mahran LG, Kafafi YA, et al. Antiulcerogenic effect of some gastrointestinally acting plant extracts and their combination. Arzneimittelforschung. 2001;51(7):545–53. https://doi.org/10.1055/s-0031-1300078.
Khayyal MT, Seif-El-Nasr M, El-Ghazaly MA, Okpanyi SN, Kelber O, Weiser D. Mechanisms involved in the gastro-protective effect of STW 5 (Iberogast) and its components against ulcers and rebound acidity. Phytomedicine. 2006;13(Suppl 5):56–66.
Abdel-Aziz H, Wadie W, Zaki HF, Müller J, Kelber O, Efferth T, et al. Novel sequential stress model for functional dyspepsia: Efficacy of the herbal preparation STW5. Phytomedicine. 2015;22(5):588–95.
Acknowledgements
The authors wish to acknowledge with deep appreciation Bayer Consumer Health, Steigerwald Arzneimittelwerk GmbH, Darmstadt, Germany, for funding this work as well as the help of Dr. Dalia H. Ragab in initiating the study.
Funding
This work was supported by Bayer Consumer Health, Steigerwald Arzneimittelwerk GmbH, Darmstadt, Germany.
Author information
Authors and Affiliations
Contributions
NFA, WW, HA, SR, RMA, LAA, and MTK contributed conception and design of the study; SSM, NFA, and WW performed the experiments, SSM and NFA performed the statistical analysis; SSM, NFA and MTK wrote the first draft of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Animal experimental procedures were approved by the institutional Ethical Committee for Animal Experimentation at the Faculty of Pharmacy, Cairo University, Cairo, Egypt, approval number (PT 1769) following the guidelines laid out in the Guide for the Care and Use of Laboratory Animals, National Academy of Science.
Consent for publication
Not applicable.
Competing interests
The authors RMA, SR and HA were employed by Bayer Consumer Health, Darmstadt, Germany. They were kind enough to consent to the proposed study presented to the company and to help in revising the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary data.
Effect of STW 5 (5 mL/Kg) on the microbiome in healthy rats. Microbial population concentrations are expressed as g, mg, μg, ng, or pg per gram of feces (indicated for each microbial population) and calculated taking 16S rRNA gene copy number into consideration as detailed in methods section. Data represented as means 5 ± standard deviation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mohamed, S.S., Abdeltawab, N.F., Wadie, W. et al. Effect of the standard herbal preparation, STW5, treatment on dysbiosis induced by dextran sodium sulfate in experimental colitis. BMC Complement Med Ther 21, 168 (2021). https://doi.org/10.1186/s12906-021-03337-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-021-03337-8