Abstract
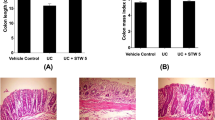
Although the pathogenic mechanisms of inflammatory bowel diseases are not fully understood, colonic microbiota may affect the induction of colonic inflammation, and some probiotics and prebiotics have been reported to suppress colitis. The inhibitory effects of brown rice fermented by Aspergillus oryzae (FBRA), a fiber-rich food, on the induction of acute colitis by dextran sulfate sodium (DSS) were examined. Feeding a 5% and 10% FBRA-containing diet significantly decreased the ulcer and erosion area in the rat colon stained with Alcian blue. In another experiment, 10% FBRA feeding decreased the ulcer index (percentage of the total length of ulcers in the full length of the colon) and colitis score, which were determined by macroscopic observation. It also decreased myeloperoxidase activity in the colonic mucosa. Viable cell numbers of Lactobacillus in the feces decreased after DSS administration and was reversely correlated with severity of colitis, while the cell number of Enterobacteriaceae increased after DSS treatment and was positively correlated with colitis severity. These results indicate that FBRA has a suppressive effect on the induction of colitis by DSS and suggest FBRA-mediated modification of colonic microbiota.



Similar content being viewed by others
References
Campieri M, Gionchetti P (2001) Bacteria as the cause of ulcerative colitis. Gut 48:132–135
Fiocchi C (2003) Microbial factors in the pathogenesis of IBD. Biosci Microflora 22:5–14
Tlaskalova-Hogenova H et al (2004) Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett 93:97–108
Danese S, Sans M, Fiocchi C (2004) Inflammatory bowel disease: the role of environmental factors. Autoimmun Rev 3:394–400
Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, Jian R, Dore J (2003) Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut 52:237–242
Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Bauke V, Ortner M, Weber J, Hoffmann U, Schreiber S, Dietel M, Lochs H (2002) Mucosal flora in inflammatory bowel disease. Gastroenterol 122:44–54
Madsen KL, Doyle JS, Tavernini MM, Jewell LD, Rennie RP, Fedorak RN (2000) Antibiotic therapy attenuates colitis in interleukin 10 gene-deficient mice. Gastroenterol 118:1094–1105
Linskens RK, Huijsdens XW, Savelkoul PHM, Vandenbroucke-Grauls CMJE, Meuwissen SGM (2001) The bacterial flora in inflammatory bowel disease: current insights in pathogenesis and the influence of antibiotics and probiotics. Scand J Gastroenterol 36(suppl 234):29–40
Tamura K, Hori K (2003) Fecal flora of inflammatory bowel disease. Clin Gastroenterol 18:829–835
Bullock NR, Booth JC, Gibson GR (2004) Comparative composition of bacteria in the human intestinal microflora during remission and active ulcerative colitis. Curr Issues Intest Microbiol 5:59–64
Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, FÖlsch UR, Timmis KN, Schreiber S (2004) Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 53:685–693
Ding L-A, Li J-S (2003) Gut in diseases: physiological elements and their clinical significance. World J Gastroenterol 9:2385–2389
Hawrelak JA, Myers SP (2004) The causes of intestinal dysbiosis: a review. Altern Med Rev 9:180–197
Marteau P, Lepage P, Mangin I, Suau A, Dore J, Pochart P, Seksik P (2004) Review article: gut flora and inflammatory bowel disease. Aliment Pharmacol Ther Suppl 4:18–23
Galvez J, Rodriguez-Cabezas ME, Zarzuelo A (2005) Effects of dietary fiber on inflammatory bowel disease. Mol Nutr Food Res 49:601–608
Kanauchi O, Matsumoto Y, Matsumura M, Fukuoka M, Bamba T (2005) The beneficial effects of microflora, especially obligate anaerobes, and their products on the colonic environment in inflammatory bowel disease. Curr Pharm Des 11:1047–1053
Guarner F, Malangelada JR (2003) Role of bacteria in experimental colitis. Best Pract Res Clin Gastroenterol 17: 793–804
Jaskari J, Kontula P, Siitonen A, Jousimies-Somer H, Mattila-Sandholm T, Poutanen K (1998) Oat (-glucan and xylan hydrolysates as selective substrates for Bifidobacterium and Lactobacillus strains. Appl Microbiol Biotechnol 49:175–181
Charalampopoulos D, Wang R, Pandiella SS, Webb C (2002) Application of cereals and cereal components in functional foods. A review. Int J Food Microbiol 79:131–141
Hsu C-K,Liao J-W, Chung Y-C, Hsieh C-P, Chan Y-C (2004) Xylooligosaccharides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. Nutr Cancer 134:1523–1528
Gomez-Alarcon RA, Dudas C, Huber JT (1990) Influence of cultures of Aspergillus oryzae on rumen and total tract digestibility of dietary components. J Dairy Sci 73:703–710
Elson CO (1999) Experimental models of intestinal inflammation. New insights into mechanisms of mucosal homeostasis. In: Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR (eds) Mucosal Immunology. Academic Press, pp 1007–1024
Hagiwara M, Kataoka K, Arimochi H, Kuwahara T, Ohnishi Y (2004) Role of unbalanced growth of Gram-negative bacteria in ileal ulcer formation in rats treated with a nonsteroidal anti-inflammatory drug. J Med Invest 51:43–51
Kinouchi T, Kataoka K, Shan R-B, Nakayama H, Uejima M, Shimono K, Kuwahara T, Akimoto S, Hiraoka I, Ohnishi Y (1998) Culture supernatants of Lactobacillus acidophilus and Bifidobacterium adolescentis repress ileal ulcer formation in rats treated with a nonsteroidal anti-inflammatory drug by suppressing unbalanced growth of aerobic bacteria and lipid peroxidation. Microbiol Immunol 42:347–355
Kimura I, Nagahama S, Kawasaki M, Kataoka M, Sato M (1996) Study on the experimental ulcerative colitis (UC) model induced by dextran sulfate sodium (DSS) in rats (3). Folia Pharmacol Jpn 108:259–266
Krawisz JE, Sharon P, Stenson WF (1984) Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterol 87:1344–1350
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mitsuoka T, Hayakawa K, Kitamura N (1974) Die faekal flora bei menschen. II. Mitteilung: die Zuzammensetzung der Bifidobakterien-flora der vershiedenen altersgruppen. Zentralblatt fur Bakteriologie und Hygiene, Abteilung 1. Originale 226:469–478
Mitsuoka T, Sega T, Yamamoto S (1965) Eine verbesserte methodic der darmflora von menschen und tieren. Zentralblatt fur Bakteriologie und Hygiene, Abteilung 1. Originale 195:455–469
Somasundaram S, Sigthorsson G, Simpson RJ, Watts J, Jacob M, Tavares IA, Rafi S, Roseth A, Foster R, Price AB, Wrigglesworth JM, Bjarnason I (2000) Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat. Aliment Pharmacol Ther 14:639–650
Ni J, Chen S-F, Hollander D (1996) Effects of dextran sulphate sodium on intestinal epithelial cells and intestinal lymphocytes. Gut 39:234–241
Schuppler M, LÖtzsch K, Waidmann M, Autenrieth IB (2004)An abundance of Escherichia coli is harbored by the mucosa-associated bacterial flora of interleukin-2-deficient mice. Infect Immun 72:1983–1990
Ferla KL, Seegert D, Schreiber S (2004) Activation of NF-κB in intestinal epithelial cells by E. coli strains isolated from the colonic mucosa of IBD patients. Int J Colorectal Dis 19:334–342
Matsuda H, Fujiyama Y, Andoh A, Ushijima T, Kajinami T, Bamba T (2000) Characterization of antibody responses against rectal mucosa-associated bacterial flora in patients with ulcerative colitis. J Gastroenterol Hepatol 15:61–68
Haller D, Holt L, Parlesak A, Zanga J, Bauerlein A, Sartor RB, Jobin C (2004) Differential effect of immune cells on non-pathogenic gram-negative bacteria-induced nuclear factor-kappaB activation and pro-inflammatory gene expression in intestinal epithelial cells. Immunology 112:310–320
Lan JG, Cruickshank SM, Singh JC, Farrar M, Lodge JP, Felsburg PJ, Carding SR (2005) Different cytokine response of primary colonic epithelial cells to commensal bacteria. World J Gastroenterol 11:3375–3384
Marteau P, Seksik P, Lepage P, Dore J (2004) Cellular and physiological effects of probiotics and prebiotics. Mini Rev Med Chem 4:889–896
Sherman PM, Johnson-Henry KC, Yeung HP, Ngo PSC, Goulet J, Tompkins TA (2005) Probiotics reduce enterohemorrhagic Escherichia coli O157: H7- and enteropathogenic E. coli O127: H6-induced changes in polarized T84 epithelial cell monolayers by reducing bacterial adhesion and cytoskelital rearrangements. Infect Immun 73:5183–5188
Hart AL, Lammers K, Brigidi P, Vitali B, Rizzello F, Gionchetti P, Campieri M, Kamm MA, Knight SC, Stagg AJ (2004) Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut 53:1602–1609
Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C (2001) Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121:580–591
Zoetendal EG, von Wright A, Vilpponen-Salmela T et al (2002) Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Environ Microbiol 68:3401–3407
Langlands SJ, Hopkins MJ, Coleman N, Cummings JH (2004) Prebiotic carbohydrates modify the mucosa-associated microflora of the human large bowel. Gut 53:1610–1616
Roediger WE (1980) The colonic epithelium in ulcerative colitis: an energy-deficiency disease? Lancet 2:712–715
Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C (2000) The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology 118:724–734
Katayama M, Yoshimi N, Yamada Y, Sakata K, Kuno T, Yoshida K, Qiao Z, Vihn P-Q, Iwasaki T, Kobayashi H, Mori H (2002) Preventive effect of fermented brown rice and rice bran against colon carcinogenesis in male F344 rats. Oncol Rep 9:817–822
Takasuga T, Senthilkumar K, Takemori H, Ohi E, Tsuji H, Nagayama J (2004) Impact of FEBRA (fermented brown rice with Aspergillus oryzae) intake and concentrations of PCDDs, PCDFs and PCBs in blood of humans from Japan. Chemosphere 57:1409–1426
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kataoka, K., Ogasa, S., Kuwahara, T. et al. Inhibitory Effects of Fermented Brown Rice on Induction of Acute Colitis by Dextran Sulfate Sodium in Rats. Dig Dis Sci 53, 1601–1608 (2008). https://doi.org/10.1007/s10620-007-0063-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-007-0063-3