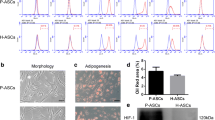
Abstract
Adipose tissue-derived stem cells (ADSCs) are capable of multipotential differentiation and express several angiogenic, anti-apoptotic and immunomodulatory markers. These features make adipose tissue as a promising source of stem cells for regenerative medicine. However, for efficient translational use, culture-induced changes in the gene expression profile and resistance of the ADSCs to ischemic environment should be taken into consideration. We compared the expression of some clinically important markers between the unpassaged and third-passaged ADSCs by RT-PCR, qPCR and flow cytometry. Our results demonstrated that the embryonic stem cell (ESC)-specific markers were expressed in the unpassaged ADSCs but were downregulated after three passages. The expression of stemness-related genes, TGFB and FGF2, was upregulated while FGF4 and LIF were downregulated after three passages. The expression of angiogenic genes in the third-passaged ADSCs was higher than the unpassaged cells. Epithelial-mesenchymal transition (EMT) markers were either expressed in the third-passaged ADSCs or significantly upregulated after three passages. In contrast, cell cycle inhibitors, CDKN1A and TP53, were downregulated with early subcultures. The unpassaged and third-passaged ADSCs showed nearly similar resistance to oxidative stress, hypoxia and serum deprivation. In conclusion, the primary cultures of human adipose tissue contain a subpopulation of cells expressing ESC-specific genes and proteins, but the expression of these pluripotency markers subsides rapidly in standard mesenchymal stem cell culture medium. The expression of angiogenic and EMT markers also varies with early subcultures. Altogether, early-passaged ADSCs may be better choices for transplantation therapy of injured tissues, especially after ischemic conditions.








Similar content being viewed by others
References
Ambrosetti DC, Basilico C, Dailey L (1997) Synergistic activation of the fibroblast growth factor 4 enhancer by Sox2 and Oct-3 depends on protein-protein interactions facilitated by a specific spatial arrangement of factor binding sites. Mol Cell Biol 17:6321–6329
Armesilla-Diaz A, Elvira G, Silva A (2009) p53 regulates the proliferation, differentiation and spontaneous transformation of mesenchymal stem cells. Exp Cell Res 315:3598–3610. doi:10.1016/j.yexcr.2009.08.004
Atlasi Y, Mowla SJ, Ziaee SAM, Gokhale PJ, Andrews PW (2008) OCT4 spliced variants are differentially expressed in human pluripotent and nonpluripotent cells. Stem Cells 26:3068–3074
Baek SJ, Kang SK, Ra JC (2011) In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp Mol Med 43:596–603. doi:10.3858/emm.2011.43.10.069
Chew JL, Loh YH, Zhang W, Chen X, Tam WL, Yeap LS, Li P, Ang YS, Lim B, Robson P, Ng HH (2005) Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol 25:6031–6046. doi:10.1128/MCB.25.14.6031-6046.2005
Chiotaki R, Polioudaki H, Theodoropoulos PA (2016) Stem cell technology in breast cancer: current status and potential applications. Stem Cells Cloning Adv Appl 9:17–29. doi:10.2147/SCCAA.S72836
Daheron L, Opitz SL, Zaehres H, Lensch MW, Andrews PW, Itskovitz-Eldor J, Daley GQ (2004) LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells 22:770–778. doi:10.1634/stemcells.22-5-770
De Becker A, Van HummelenP, Bakkus M, Vande Broek I, De WeverJ, De WaeleM, Van RietI (2007) Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica 92:440–449
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. Int Soc Cell Ther Position Statement Cytother 8:315–317. doi:10.1080/14653240600855905
Endoh H, Kaneko T, Doi K, Takahashi E (2000) Improved cardiac contractile functions in hypoxia-reoxygenation in rats treated with low concentration Co(2+). Am J Physiol Heart Circ Physiol 279:H2713–H2719
Eyssen-Hernandez R, Ladoux A, Frelin C (1996) Differential regulation of cardiac heme oxygenase-1 and vascular endothelial growth factor mRNA expressions by hemin, heavy metals, heat shock and anoxia. FEBS Lett 382:229–233
Farashahi Yazd E, Rafiee MR, Soleimani M, Tavallaei M, Salmani MK, Mowla SJ (2011) OCT4B1, a novel spliced variant of OCT4, generates a stable truncated protein with a potential role in stress response. Cancer Lett 309:170–175
Fisher JW, Langston JW (1968) Effects of testosterone, cobalt and hypoxia on erythropoietin production in the isolated perfused dog kidney. Ann NY Acad Sci 149:75–87
Friedman E, Gold LI, Klimstra D, Zeng ZS, Winawer S, Cohen A (1995) High levels of transforming growth factor beta 1 correlate with disease progression in human colon cancer Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology 4:549–554
Greco SJ, Liu K, Rameshwar P (2007) Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem Cells 25:3143–3154. doi:10.1634/stemcells.2007-0351
Gupta DK, Singh N, Sahu DK (2014) TGF-beta mediated crosstalk between malignant hepatocyte and tumor microenvironment in hepatocellular carcinoma. Cancer Growth Metastasis 7:1–8. doi:10.4137/CGM.S14205
Halaban R, Kwon BS, Ghosh S, Delli Bovi P, Baird A (1988) bFGF as an autocrine growth factor for human melanomas. Oncog Res 3:177–186
Heneidi S, Simerman AA, Keller E, Singh P, Li X, Dumesic DA, Chazenbalk G (2013) Awakened by cellular stress: isolation and characterization of a novel population of pluripotent stem cells derived from human adipose tissue. PloS ONE 8:e64752. doi:10.1371/journal.pone.0064752
Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE (2006) Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 24:1030–1041. doi:10.1634/stemcells.2005-0319
Huang YC, Yang ZM, Chen XH, Tan MY, Wang J, Li XQ, Xie HQ, Deng L (2009) Isolation of mesenchymal stem cells from human placental decidua basalis and resistance to hypoxia and serum deprivation. Stem Cell Rev 5:247–255. doi:10.1007/s12015-009-9069-x
Hwang ST, Kang SW, Lee SJ, Lee TH, Suh W, Shim SH, Lee DR, Taite LJ, Kim KS, Lee SH (2010) The expansion of human ES and iPS cells on porous membranes and proliferating human adipose-derived feeder cells. Biomaterials 31:8012–8021. doi:10.1016/j.biomaterials.2010.07.031
Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA (2006) Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem 99:1285–1297. doi:10.1002/jcb.20904
James D, Levine AJ, Besser D, Hemmati-Brivanlou A (2005) TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development 132:1273–1282. doi:10.1242/dev.01706
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41–49. doi:10.1038/nature00870
Kalluri R (2009) EMT: when epithelial cells decide to become mesenchymal-like cells. J Clin Investig 119:1417–1419. doi:10.1172/JCI39675
Kim JS, Kwon D, Hwang ST, Lee DR, Shim SH, Kim HC, Park H, Kim W, Han MK, Lee SH (2013) hESC expansion and stemness are independent of connexin forty-three-mediated intercellular communication between hESCs and hASC feeder cells. PLoS ONE 8:e69175. doi:10.1371/journal.pone.0069175
Krock BL, Skuli N, Simon MC (2011) Hypoxia-induced angiogenesis: good and evil. Genes Cancer 2:1117–1133. doi:10.1177/1947601911423654
Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak MZ (2006) A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia 20:857–869. doi:10.1038/sj.leu.2404171
Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, Goda M, Akashi H, Inutsuka A, Niwa A, Shigemoto T, Nabeshima Y, Nakahata T, Nabeshima Y, Fujiyoshi Y, Dezawa M (2010) Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci USA 107:8639–8643. doi:10.1073/pnas.0911647107
Kwon MJ, Kang SJ, Park YI, Yang YH, Bang SI, Park YH, So B, Cho MH, Kang HG (2015) Hepatic differentiation of human adipose tissue-derived mesenchymal stem cells and adverse effects of arsanilic acid and acetaminophen during in vitro hepatic developmental stage. Cell Biol Toxicol 31:149–159. doi:10.1007/s10565-015-9300-2
Liu GY, Jiang XX, Zhu X, He WY, Kuang YL, Ren K, Lin Y, Gou X (2015) ROS activates JNK-mediated autophagy to counteract apoptosis in mouse mesenchymal stem cells in vitro. Acta Pharmacol Sin 36:1473–1479. doi:10.1038/aps.2015.101
Mattar P, Bieback K (2015) Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells. Front Immunol 6:560. doi:10.3389/fimmu.2015.00560
Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S (2003) The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113:631–642
Moll UM, Wolff S, Speidel D, Deppert W (2005) Transcription-independent pro-apoptotic functions of p53. Curr Opin Cell Biol 17:631–636. doi:10.1016/j.ceb.2005.09.007
Momin EN, Vela G, Zaidi HA, Quinones-Hinojosa A (2010) The oncogenic potential of mesenchymal stem cells in the treatment of cancer: directions for future research. Curr Immunol Rev 6:137–148. doi:10.2174/157339510791111718
Nam JS, Kang HM, Kim J, Park S, Kim H, Ahn CW, Park JO, Kim KR (2014) Transplantation of insulin-secreting cells differentiated from human adipose tissue-derived stem cells into type 2 diabetes mice. Biochem Biophys Res Commun 443:775–781. doi:10.1016/j.bbrc.2013.10.059
Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Schöler H, Smith A (1998) Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95:379–391
Nishimoto M, Fukushima A, Okuda A, Muramatsu M (1999) The gene for the embryonic stem cell coactivator UTF1 carries a regulatory element which selectively interacts with a complex composed of Oct-3/4 and Sox-2. Mol Cell Biol 19:5453–5465
Peroni D, Scambi I, Pasini A, Lisi V, Bifari F, Krampera M, Rigotti G, Sbarbati A, Galiè M (2008) Stem molecular signature of adipose-derived stromal cells. Exp Cell Res 314:603–615. doi:10.1016/j.yexcr.2007.10.007
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucl Acids Res 30:e36
Piret JP, Mottet D, Raes M, Michiels C (2002) CoCl2, a chemical inducer of hypoxia-inducible factor-1, and hypoxia reduce apoptotic cell death in hepatoma cell line HepG2. Ann NY Acad Sci 973:443–447
Piret JP, Lecocq C, Toffoli S, Ninane N, Raes M, Michiels C (2004) Hypoxia and CoCl2 protect HepG2 cells against serum deprivation- and t-BHP-induced apoptosis: a possible anti-apoptotic role for HIF-1. Exp Cell Res 295:340–349. doi:10.1016/j.yexcr.2004.01.024
Prusa AR, Marton E, Rosner M, Bernaschek G, Hengstschläger M (2003) Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Hum Reprod 18:1489–1493
Rasmussen JG, Frøbert O, Holst-Hansen C, Kastrup J, Baandrup U, Zachar V, Fink T, Simonsen U (2014) Comparison of human adipose-derived stem cells and bone marrow-derived stem cells in a myocardial infarction model. Cell Transplant 23:195–206. doi:10.3727/096368912X659871
Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL (2004) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109:1292–1298. doi:10.1161/01.CIR.0000121425.42966.F1
Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, Robson P (2005) Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem 280:24731–24737. doi:10.1074/jbc.M502573200
Rykala J, Przybylowska K, Majsterek I, Pasz-Walczak G, Sygut A, Dziki A, Kruk-Jeromin J (2011) Angiogenesis markers quantification in breast cancer and their correlation with clinicopathological prognostic variables. Pathol Oncol Res 17:809–817. doi:10.1007/s12253-011-9387-6
Sawada K, Takedachi M, Yamamoto S, Morimoto C, Ozasa M, Iwayama T, Lee CM, Okura H, Matsuyama A, Kitamura M, Murakami S (2015) Trophic factors from adipose tissue-derived multi-lineage progenitor cells promote cytodifferentiation of periodontal ligament cells. Biochem Biophys Res Commun 464:299–305. doi:10.1016/j.bbrc.2015.06.147
Schaffler A, Buchler C (2007) Concise review: adipose tissue-derived stromal cells-basic and clinical implications for novel cell-based therapies. Stem Cells 25:818–827. doi:10.1634/stemcells.2006-0589
Simerman AA, Dumesic DA, Chazenbalk GD (2014) Pluripotent muse cells derived from human adipose tissue: a new perspective on regenerative medicine and cell therapy. Clin Transl Med 3:12. doi:10.1186/2001-1326-3-12
Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A (2006) Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells 24:1254–1264. doi:10.1634/stemcells.2005-0271
Taha MF, Hedayati V (2010) Isolation, identification and multipotential differentiation of mouse adipose tissue-derived stem cells. Tissue Cell 42:211–216. doi:10.1016/j.tice.2010.04.003
Taha MF, Javeri A, Rohban S, Mowla SJ (2014) Upregulation of pluripotency markers in adipose tissue-derived stem cells by miR-302 and leukemia inhibitory factor. Biomed Res Int 2014:941486. doi:10.1155/2014/941486
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890. doi:10.1016/j.cell.2009.11.007
Triantafyllou A, Liakos P, Tsakalof A, Georgatsou E, Simos G, Bonanou S (2006) Cobalt induces hypoxia-inducible factor-1alpha (HIF-1alpha) in HeLa cells by an iron-independent, but ROS-, PI-3K- and MAPK-dependent mechanism. Free Radic Res 40:847–856. doi:10.1080/10715760600730810
Vallier L, Alexander M, Pedersen RA (2005) Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci 118:4495–4509. doi:10.1242/jcs.02553
Voutsadakis IA (2015) The network of pluripotency, epithelial–mesenchymal transition, and prognosis of breast cancer. Breast Cancer 7:303–319. doi:10.2147/BCTT.S71163
Wang ZX, Teh CH, Kueh JL, Lufkin T, Robson P, Stanton LW (2007) Oct4 and Sox2 directly regulate expression of another pluripotency transcription factor, Zfp206, in embryonic stem cells. J Biol Chem 282:12822–12830. doi:10.1074/jbc.M611814200
Wei H, Li Z, Hu S, Chen X, Cong X (2010) Apoptosis of mesenchymal stem cells induced by hydrogen peroxide concerns both endoplasmic reticulum stress and mitochondrial death pathway through regulation of caspases, p38 and JNK. J Cell Biochem 111:967–978. doi:10.1002/jcb.22785
Wu K, Xu W, You Q, Guo R, Feng J, Zhang C, Wu W (2012) Increased expression of heat shock protein 90 under chemical hypoxic conditions protects cardiomyocytes against injury induced by serum and glucose deprivation. Int J Mol Med 30:1138–1144. doi:10.3892/ijmm.2012.1099
Wynn RF, Hart CA, Corradi-Perini C, O’Neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I (2004) A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 104:2643–2645. doi:10.1182/blood-2004-02-0526
Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA (2002) BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol 20:1261–1264. doi:10.1038/nbt761
Yang Z, Yang C, Xiao L, Liao X, Lan A, Wang X, Guo R, Chen P, Hu C, Feng J (2011) Novel insights into the role of HSP90 in cytoprotection of H2S against chemical hypoxia-induced injury in H9c2 cardiac myocytes. Int J Mol Med 28:397–403. doi:10.3892/ijmm.2011.682
Ybarra J, Behrooz A, Gabriel A, Koseoglu MH, Ismail-Beigi F (1997) Glycemia-lowering effect of cobalt chloride in the diabetic rat: increased GLUT1 mRNA expression. Mol Cell Endocrinol 133:151–160
Ying QL, Nichols J, Chambers I, Smith A (2003) BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115:281–292
Yuan H, Corbi N, Basilico C, Dailey L (1995) Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes Dev 9:2635–2645
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295. doi:10.1091/mbc.E02-02-0105
Acknowledgements
This study was financially supported by Iran National Science Foundation (INSF) (Grant No. 78040521).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Faghih, H., Javeri, A. & Taha, M.F. Impact of early subcultures on stemness, migration and angiogenic potential of adipose tissue-derived stem cells and their resistance to in vitro ischemic condition. Cytotechnology 69, 885–900 (2017). https://doi.org/10.1007/s10616-017-0104-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-017-0104-5