Abstract
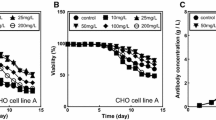
Controlling cell proliferation during cell culturing is an effective way to improve the production yield in mammalian cell culture. We examined the effect of temperature shifts (TS) under pH control conditions in Chinese hamster ovary cells. When we shifted the culture temperature from 37 °C to 31 °C before a stationary phase at pH 6.8 (TS/pH 6.8), cell viability remained high, and the final human monoclonal antibody (hMab) concentration increased to 2.3 times that in the culture remaining at 37 °C. However, there were no significant effects on the cell viability or production yield with the same TS at pH 7.0 (TS/pH 7.0). The average specific hMab productivity and mRNA level of TS/pH 7.0 were the same as that of TS/pH 6.8. The control of cell growth by the TS or the addition of rapamycin was effective in the maintenance of cell viability, but there was no significant increase of the average specific hMab productivity in the culture where cell proliferation was controlled with rapamycin. The hMab mRNA concentration decreased to 55%–65% at a 37 °C culture with the addition of actinomycin D. In contrast, actinomycin D did not affect the mRNA level in the TS culture. This result suggested that the increase in the mRNA level in the TS condition was caused by an increase in mRNA stability. In this study, we show that TS can produce two unrelated effects: a prolongation of cell longevity and an improvement in mRNA stability.




Similar content being viewed by others
Abbreviations
- CHO cell:
-
Chinese hamster ovary cell
- HC:
-
Heavy chain
- hMab:
-
Human monoclonal antibody
- IVCD:
-
Integral of viable cell density
- LC:
-
Light chain
- TS:
-
Temperature shift
- TS/pH 6.8:
-
Temperature shift cultivation, pH 6.8
- TS/pH 7.0:
-
Temperature shift cultivation, pH 7.0
- VCD:
-
Viable cell density
- 37 °C/pH 6.8:
-
Culture at 37 °C, pH 6.8
- 37 °C/pH 7.0:
-
Culture at 37 °C, pH 7.0
References
Balcarcel RR, Stephanopoulos G (2001) Rapamycin reduces hybridoma cell death and enhances monoclonal antibody production. Biotechnol Bioeng 76:1–10
Bloemkolk J, Gray MR, Merchant F, Mosmann TR (1992) Effect of temperature on hybridoma cell cycle and Mab production. Biotechnol Bioeng 40:427–431
Ducommun P, Ruffieux P, Kadouri A, Stockar U, Marison I (2002) Monitoring of temperature effects on animal cell metabolism in a packed bed process. Biotechnol Bioeng 77:838–842
Fox SR, Patel UA, Yap MGS, Wang DC (2004) Maximizing interferon-γ production by chinese hamster ovary cells through temperature shift optimization: Experimental and modeling. Biotechnol Bioeng 85:177–184
Giard DJ, Fleischaker RJ, Fabricant M (1982) Effect of temperature on the production of human fibroblast interferon. Proc Soc Exp Biol Med 170:155–159
Hendrick H, Winnepenninckx P, Abdelkafi C, Vandeputte O, Cherlet M, Marique T, Renemann G, Loa A, Kretzmer G, Werenne J (2001) Increased productivity of recombinant tissular plasminogen activator (t-PA) by butyrate and shift of temperature: a cell cycle phases analysis. Cytotechnology 36:71–83
Hung DT, Jamison TF, Schreiber SL (1996) Understanding and controlling the cell cycle with natural products. Chem Biol 3:623–639
Kaufmann H, Mazur X, Fussenegger M, Bailey JE (1999) Influence of low temperature on productivity, proteome and protein phosphorylation of CHO cells. Biotechnol Bioeng 63:573–582
Kaufmann H, Mazur X, Marone R, Bailey JE (2001) Comparative analysis of two controlled proliferation strategies regarding product quality, influence on tetracycline-regulated gene expression, and productivity. Biotechnol Bioeng 72:592–602
Nishiyama H, Itoh K, Kaneko Y, Kishishita M, Yoshida O, Fujita J (1997) A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J Cell Biol 137:899–908
Paul A, Brune K (2002) Stabilization of gene expression profiles in blood after phlebotomy. Clin Chem 48:2251–2253
Ryu JS, Kim TK, Chung JY, Lee GM (2000) Osmoprotective effect of glycine betaine on foreign protein production in hyperosmotic recombinant chinese hamster ovary cell cultures differs among cell lines. Biotechnol Bioeng 70:167–175
Reff ME (1997) Impaired dominant selectable marker sequence and intronic insertion strategies for enhancement of expression of gene product and expression vector systems comprising same. US Patent 5.648.267
Reuveny S, Kim YJ, Kemp CW, Shiloach J (1993) Effect of temperature and oxygen on cell growth and recombinant protein production in insect cell cultures. Applied Microbiol Biotechnol 38:619–623
Sauer PW, Burky JE, Wesson MC, Sternard HD, Qu L (2000) A high-yielding, generic-fed batch cell culture process for production of recombinant antibodies. Biotechnol Bioeng 67:585–597
Schatz SM, Kerschbaumer RJ, Gerstenbauer G, Kral M, Dorner F, Scheiflinger F (2003) Higher expression of Fab antibody fragments in a CHO cell line at reduced temperature. Biotechnol Bioeng 84:433–438
Sureshkumar GK, Mutharasan R (1991) The influence of temperature on a mouse–mouse hybridoma growth and monoclonal antibody production. Biotechnol Bioeng 37:292–295
Varley J, Birch J (1999) Reactor design for large scale suspension animal cell culture. Cytotechnology 29:177–205
Watanabe S, Shuttleworth J, Al-Rubeai M (2002) Regulation of cell cycle and productivity in NS0 cells by the over-expression of p21CIP1. Biotechnol Bioeng 77:1–7
Weidemann R, Ludwig A, Kretzmer G (1994) Low temperature cultivation – A step towards process optimisation. Cytotechnology 15:111–116
Yoon SK, Song JY, Lee GM (2003) Effect of low temperature on specific productivity, transcription level, and heterogeneity of erythropoietin in chinese hamster ovary cells. Biotechnol Bioeng 82:289–298
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oguchi, S., Saito, H., Tsukahara, M. et al. pH Condition in temperature shift cultivation enhances cell longevity and specific hMab productivity in CHO culture. Cytotechnology 52, 199–207 (2006). https://doi.org/10.1007/s10616-007-9059-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-007-9059-2